Safety and Efficacy of Antithrombotic Strategies in Patients With Atrial Fibrillation Undergoing Percutaneous Coronary Intervention: A Network Meta-analysis of Randomized Controlled Trials
- PMID: 31215979
- PMCID: PMC6584885
- DOI: 10.1001/jamacardio.2019.1880
Safety and Efficacy of Antithrombotic Strategies in Patients With Atrial Fibrillation Undergoing Percutaneous Coronary Intervention: A Network Meta-analysis of Randomized Controlled Trials
Abstract
Importance: The antithrombotic treatment of patients with atrial fibrillation (AF) and coronary artery disease, in particular with acute coronary syndrome (ACS) and/or percutaneous coronary intervention (PCI), poses a significant treatment dilemma in clinical practice.
Objective: To study the safety and efficacy of different antithrombotic regimens using a network meta-analysis of randomized controlled trials in this population.
Data sources: PubMed, EMBASE, EBSCO, and Cochrane databases were searched to identify randomized controlled trials comparing antithrombotic regimens.
Study selection: Four randomized studies were included (n = 10 026; WOEST, PIONEER AF-PCI, RE-DUAL PCI, and AUGUSTUS).
Data extraction and synthesis: The Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines were used in this systematic review and network meta-analysis between 4 regimens using a Bayesian random-effects model. A pre hoc statistical analysis plan was written, and the review protocol was registered at PROSPERO. Data were analyzed between November 2018 and February 2019.
Main outcomes and measures: The primary safety outcome was Thrombolysis in Myocardial Infarction (TIMI) major bleeding; secondary safety outcomes were combined TIMI major and minor bleeding, trial-defined primary bleeding events, intracranial hemorrhage, and hospitalization. The primary efficacy outcome was trial-defined major adverse cardiovascular events (MACE); secondary efficacy outcomes were individual components of MACE.
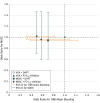
Results: The overall prevalence of ACS varied from 28% to 61%. The mean age ranged from 70 to 72 years; 20% to 29% of the trial population were women; and most patients were at high risk for thromboembolic and bleeding events. Compared with a regimen of vitamin K antagonist (VKA) plus dual antiplatelet therapy (DAPT; P2Y12 inhibitor plus aspirin), the odds ratios (ORs) for TIMI major bleeding were 0.58 (95% CI, 0.31-1.08) for VKA plus P2Y12 inhibitor, 0.49 (95% CI, 0.30-0.82) for non-VKA oral anticoagulant (NOAC) plus P2Y12 inhibitor, and 0.70 (95% CI, 0.38-1.23) for NOAC plus DAPT. Compared with VKA plus DAPT, the ORs for MACE were 0.96 (95% CI, 0.60-1.46) for VKA plus P2Y12 inhibitor, 1.02 (95% CI, 0.71-1.47) for NOAC plus P2Y12 inhibitor, and 0.94 (95% CI, 0.60-1.45) for NOAC plus DAPT.
Conclusions and relevance: A regimen of NOACs plus P2Y12 inhibitor was associated with less bleeding compared with VKAs plus DAPT. Strategies omitting aspirin caused less bleeding, including intracranial bleeding, without significant difference in MACE, compared with strategies including aspirin. Our results support the use of NOAC plus P2Y12 inhibitor as the preferred regimen post-percutaneous coronary intervention for these high-risk patients with AF. A regimen of VKA plus DAPT should generally be avoided.
Conflict of interest statement
Figures


Comment in
-
Clinical Considerations Prior to Transition From Triple Antithrombotic Therapy to Dual Antithrombotic Therapy-Reply.JAMA Cardiol. 2020 Jan 1;5(1):111-112. doi: 10.1001/jamacardio.2019.4551. JAMA Cardiol. 2020. PMID: 31799984 No abstract available.
-
Clinical Considerations Prior to Transition From Triple Antithrombotic Therapy to Dual Antithrombotic Therapy.JAMA Cardiol. 2020 Jan 1;5(1):111. doi: 10.1001/jamacardio.2019.4542. JAMA Cardiol. 2020. PMID: 31799985 No abstract available.
References
-
- Dewilde WJM, Oirbans T, Verheugt FWA, et al. ; WOEST study investigators . Use of clopidogrel with or without aspirin in patients taking oral anticoagulant therapy and undergoing percutaneous coronary intervention: an open-label, randomised, controlled trial. Lancet. 2013;381(9872):1107-1115. doi:10.1016/S0140-6736(12)62177-1 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

