Tumor-proximal liquid biopsy to improve diagnostic and prognostic performances of circulating tumor cells
- PMID: 31216108
- PMCID: PMC6717761
- DOI: 10.1002/1878-0261.12534
Tumor-proximal liquid biopsy to improve diagnostic and prognostic performances of circulating tumor cells
Abstract
Circulating tumor cell (CTC) detection and numeration are becoming part of the common clinical practice, especially for breast, colon, and prostate cancer. However, their paucity in peripheral blood samples is an obstacle for their identification. Several groups have tried to improve CTC recovery rate by developing highly sensitive cellular and molecular detection methods. However, CTCs are still difficult to detect in peripheral blood. Therefore, their recovery rate could be increased by obtaining blood samples from vessels close to the drainage territories of the invaded organ, when the anatomical situation is favorable. This approach has been tested mostly during tumor resection surgery, when the vessels nearest to the tumor are easily accessible. Moreover, radiological (including echo-guided based and endovascular techniques) and/or endoscopic routes could be utilized to obtain CTC samples close to the tumor in a less invasive way than conventional biopsies. The purpose of this article is to summarize the available knowledge on CTC recovery from blood samples collected close to the tumor (i.e., in vessels located in the drainage area of the primary tumor or metastases). The relevance of such an approach for diagnostic and prognostic evaluations will be discussed, particularly for pancreatic ductal adenocarcinoma, colorectal adenocarcinoma, hepatocellular carcinoma, and non-small-cell lung cancer.
Keywords: cancer diagnostics; cancer prognosis; circulating tumor cells; liquid biopsy; vascular organ drainage.
© 2019 The Authors. Published by FEBS Press and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
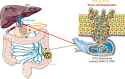
 ). Pancreatic cancer and colorectal cancer metastases in the liver (
). Pancreatic cancer and colorectal cancer metastases in the liver ( ) develop through multiple steps. Local invasion by cancer cells is followed by their intravasation into the tumor vasculature. Cancer cells then enter the porto‐mesenteric venous system as single cells or clusters that might be coated by platelets.
) develop through multiple steps. Local invasion by cancer cells is followed by their intravasation into the tumor vasculature. Cancer cells then enter the porto‐mesenteric venous system as single cells or clusters that might be coated by platelets. 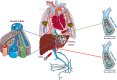
 ) and in the central vein (★) that constitute the hepatic vein system draining into the inferior vena cava. They represent the main intrahepatic and pulmonary metastatic routes (
) and in the central vein (★) that constitute the hepatic vein system draining into the inferior vena cava. They represent the main intrahepatic and pulmonary metastatic routes ( ). Blood sampling from the hepatic veins (green circle) could improve
). Blood sampling from the hepatic veins (green circle) could improve 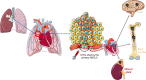
Similar articles
-
Liquid biopsy in pancreatic ductal adenocarcinoma: current status of circulating tumor cells and circulating tumor DNA.Mol Oncol. 2019 Aug;13(8):1623-1650. doi: 10.1002/1878-0261.12537. Epub 2019 Jul 30. Mol Oncol. 2019. PMID: 31243883 Free PMC article. Review.
-
Circulating Tumor Cells as a Biomarker in Pancreatic Ductal Adenocarcinoma.Cell Physiol Biochem. 2017;42(1):373-382. doi: 10.1159/000477481. Epub 2017 May 25. Cell Physiol Biochem. 2017. PMID: 28558380
-
Molecular detection of epithelial-mesenchymal transition markers in circulating tumor cells from pancreatic cancer patients: Potential role in clinical practice.World J Gastroenterol. 2019 Jan 7;25(1):138-150. doi: 10.3748/wjg.v25.i1.138. World J Gastroenterol. 2019. PMID: 30643364 Free PMC article.
-
Portal venous circulating tumor cells as a biomarker for relapse prediction in resected pancreatic cancer.Cell Mol Life Sci. 2025 Apr 10;82(1):155. doi: 10.1007/s00018-025-05669-x. Cell Mol Life Sci. 2025. PMID: 40208273 Free PMC article.
-
Circulating tumor cells: clinical validity and utility.Int J Clin Oncol. 2017 Jun;22(3):421-430. doi: 10.1007/s10147-017-1105-2. Epub 2017 Feb 25. Int J Clin Oncol. 2017. PMID: 28238187 Review.
Cited by
-
Immunological Markers, Prognostic Factors and Challenges Following Curative Treatments for Hepatocellular Carcinoma.Int J Mol Sci. 2021 Sep 24;22(19):10271. doi: 10.3390/ijms221910271. Int J Mol Sci. 2021. PMID: 34638613 Free PMC article. Review.
-
Progress and application of circulating tumor cells in non-small cell lung cancer.Mol Ther Oncolytics. 2021 May 19;22:72-84. doi: 10.1016/j.omto.2021.05.005. eCollection 2021 Sep 24. Mol Ther Oncolytics. 2021. PMID: 34514090 Free PMC article. Review.
-
Unveiling the dynamics of circulating tumor cells in colorectal cancer: from biology to clinical applications.Front Cell Dev Biol. 2024 Oct 30;12:1498032. doi: 10.3389/fcell.2024.1498032. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 39539964 Free PMC article. Review.
-
Spatial heterogeneity in epithelial to mesenchymal transition properties of circulating tumor cells associated with distant recurrence in pancreatic cancer patients.Ann Transl Med. 2020 Jun;8(11):676. doi: 10.21037/atm-20-782. Ann Transl Med. 2020. PMID: 32617296 Free PMC article.
-
Liquid biopsies in primary and secondary bone cancers.Cancer Drug Resist. 2022 Jun 21;5(3):541-559. doi: 10.20517/cdr.2022.17. eCollection 2022. Cancer Drug Resist. 2022. PMID: 36176757 Free PMC article. Review.
References
-
- Alix‐Panabières C and Pantel K (2013) Circulating tumor cells: liquid biopsy of cancer. Clin Chem 59, 110–118. - PubMed
-
- Alix‐Panabières C and Pantel K (2014) Challenges in circulating tumor cell research. Nat Rev Cancer 14, 623–631. - PubMed
-
- Alvarez Cubero MJ, Lorente JA, Robles‐Fernandez I, Rodriguez‐Martinez A, Puche JL and Serrano MJ (2017) Circulating tumor cells: markers and methodologies for enrichment and detection. Methods in Mol Biol (Clifton, NJ) 1634, 283–303. - PubMed
-
- Andree KC, Mentink A, Zeune LL, Terstappen LW, Stoecklein NH, Neves RP, Driemel C, Lampignano R, Yang L, Neubauer H et al (2018) Toward a real liquid biopsy in metastatic breast and prostate cancer: diagnostic leukapheresis increases CTC yields in a European Prospective Multicenter Study (CTCTrap). Int J Cancer 143, 2584–2591. - PMC - PubMed
-
- Ashworth TR (1869) A case of cancer in which cells similar to those in the tumors were seen in the blood after death. Aust Med J 14, 146–149.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

