Final Overall Survival of a Randomized Trial of Bevacizumab for Primary Treatment of Ovarian Cancer
- PMID: 31216226
- PMCID: PMC6879307
- DOI: 10.1200/JCO.19.01009
Final Overall Survival of a Randomized Trial of Bevacizumab for Primary Treatment of Ovarian Cancer
Abstract
Purpose: We report the final, protocol-specified analysis of overall survival (OS) in GOG-0218, a phase III, randomized trial of bevacizumab in women with newly diagnosed ovarian, fallopian tube, or primary peritoneal carcinoma.
Methods: A total of 1,873 women with incompletely resected stage III to IV disease were randomly assigned 1:1:1 to six 21-day cycles of intravenous carboplatin (area under the concentration v time curve 6) and paclitaxel (175 mg/m2) versus chemotherapy plus concurrent bevacizumab (15 mg/kg, cycles 2 to 6) versus chemotherapy plus concurrent and maintenance bevacizumab (cycles 2 to 22). Inclusion criteria included a Gynecologic Oncology Group performance status of 0 to 2 and no history of clinically significant vascular events or evidence of intestinal obstruction. OS was analyzed in the intention-to-treat population. A total of 1,195 serum and/or tumor specimens were sequenced for BRCA1/2 and damaging mutations in homologous recombination repair (HRR) genes. Intratumoral microvessel density was studied using CD31 immunohistochemistry.
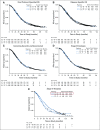
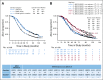
Results: Median follow-up was 102.9 months. Relative to control (n = 625), for patients receiving bevacizumab-concurrent (n = 625), the hazard ratio (HR) of death was 1.06 (95% CI, 0.94 to 1.20); for bevacizumab-concurrent plus maintenance (n = 623), the HR was 0.96 (95% CI, 0.85 to 1.09). Disease-specific survival was not improved in any arm. No survival advantage was observed after censoring patients who received bevacizumab at crossover or as second line. Median OS for stage IV bevacizumab-concurrent plus maintenance was 42.8 v 32.6 months for stage IV control (HR, 0.75; 95% CI, 0.59 to 0.95). Relative to wild type, the HR for death for BRCA1/2 mutated carcinomas was 0.62 (95% CI, 0.52 to 0.73), and for non-BRCA1/2 HRR, the HR was 0.65 (95% CI, 0.51 to 0.85). BRCA1/2, HRR, and CD31 were not predictive of bevacizumab activity.
Conclusion: No survival differences were observed for patients who received bevacizumab compared with chemotherapy alone. Testing for BRCA1/2 mutations and homologous recombination deficiency is essential.
Trial registration: ClinicalTrials.gov NCT00262847.
Figures

Comment in
-
Bevacizumab As Maintenance Treatment in Patients With Ovarian Cancer: Wait for BRCA Testing.J Clin Oncol. 2020 Jan 10;38(2):172-173. doi: 10.1200/JCO.19.02055. Epub 2019 Nov 7. J Clin Oncol. 2020. PMID: 31697587 No abstract available.
-
Bevacizumab Moonshots: An Important Outcome From the Latest Ovarian Cancer Mission.J Clin Oncol. 2020 Jan 10;38(2):171-172. doi: 10.1200/JCO.19.01912. Epub 2019 Nov 7. J Clin Oncol. 2020. PMID: 31697588 No abstract available.
-
Reply to Farolfi et al and Haines et al.J Clin Oncol. 2020 Jan 10;38(2):173-174. doi: 10.1200/JCO.19.02334. Epub 2019 Nov 7. J Clin Oncol. 2020. PMID: 31697589 No abstract available.
References
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7–34. - PubMed
-
- Tewari KS, Penson RT, Monk BJ: Ovarian cancer, in DeVita VT, Lawrence TS, Rosenberg SA (eds): DeVita, Hellman, and Rosenberg’s Cancer: Principles & Practice of Oncology (ed 11). Philadelphia, PA, Lippincott, Williams & Wilkins, 2018.
-
- Bristow RE, Tomacruz RS, Armstrong DK, et al. Survival effect of maximal cytoreductive surgery for advanced ovarian carcinoma during the platinum era: A meta-analysis. J Clin Oncol. 2002;20:1248–1259. - PubMed
-
- Rutten MJ, van Meurs HS, van de Vrie R, et al. Laparoscopy to predict the result of primary cytoreductive surgery in patients with advanced ovarian cancer: A randomized controlled trial. J Clin Oncol. 2017;35:613–621. - PubMed
-
- Eskander RN, Kauderer J, Tewari KS, et al. Correlation between surgeon’s assessment and radiographic evaluation of residual disease in women with advanced stage ovarian cancer reported to have undergone optimal surgical cytoreduction: An NRG Oncology/Gynecologic Oncology Group study. Gynecol Oncol. 2018;149:525–530. - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- U10 CA180868/CA/NCI NIH HHS/United States
- U24 CA114793/CA/NCI NIH HHS/United States
- UG1 CA233329/CA/NCI NIH HHS/United States
- U10 CA027469/CA/NCI NIH HHS/United States
- P30 CA008748/CA/NCI NIH HHS/United States
- UG1 CA233191/CA/NCI NIH HHS/United States
- UG1 CA233290/CA/NCI NIH HHS/United States
- UG1 CA189867/CA/NCI NIH HHS/United States
- UG1 CA233331/CA/NCI NIH HHS/United States
- UG1 CA233193/CA/NCI NIH HHS/United States
- U10 CA037517/CA/NCI NIH HHS/United States
- U10 CA180822/CA/NCI NIH HHS/United States
- UG1 CA233328/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

