Gut organoids: mini-tissues in culture to study intestinal physiology and disease
- PMID: 31216420
- PMCID: PMC6766612
- DOI: 10.1152/ajpcell.00300.2017
Gut organoids: mini-tissues in culture to study intestinal physiology and disease
Abstract
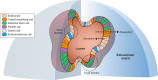
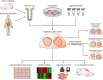
In vitro, cell cultures are essential tools in the study of intestinal function and disease. For the past few decades, monolayer cellular cultures, such as cancer cell lines or immortalized cell lines, have been widely applied in gastrointestinal research. Recently, the development of three-dimensional cultures known as organoids has permitted the growth of normal crypt-villus units that recapitulate many aspects of intestinal physiology. Organoid culturing has also been applied to study gastrointestinal diseases, intestinal-microbe interactions, and colorectal cancer. These models are amenable to CRISPR gene editing and drug treatments, including high-throughput small-molecule testing. Three-dimensional intestinal cultures have been transplanted into mice to develop versatile in vivo models of intestinal disease, particularly cancer. Limitations of currently available organoid models include cost and challenges in modeling nonepithelial intestinal cells, such as immune cells and the microbiota. Here, we describe the development of organoid models of intestinal biology and the applications of organoids for study of the pathophysiology of intestinal diseases and cancer.
Keywords: colon cancer; genetic editing; gut physiology; intestinal organoids; organoid culture.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures




Similar articles
-
Organoid Models of Human Gastrointestinal Development and Disease.Gastroenterology. 2016 May;150(5):1098-1112. doi: 10.1053/j.gastro.2015.12.042. Epub 2016 Jan 14. Gastroenterology. 2016. PMID: 26774180 Free PMC article. Review.
-
The Use of Gut Organoids: To Study the Physiology and Disease of the Gut Microbiota.J Cell Mol Med. 2025 Feb;29(4):e70330. doi: 10.1111/jcmm.70330. J Cell Mol Med. 2025. PMID: 39968926 Free PMC article. Review.
-
A High-Throughput Organoid Microinjection Platform to Study Gastrointestinal Microbiota and Luminal Physiology.Cell Mol Gastroenterol Hepatol. 2018 May 22;6(3):301-319. doi: 10.1016/j.jcmgh.2018.05.004. eCollection 2018. Cell Mol Gastroenterol Hepatol. 2018. PMID: 30123820 Free PMC article.
-
Modeling infectious diseases and host-microbe interactions in gastrointestinal organoids.Dev Biol. 2016 Dec 15;420(2):262-270. doi: 10.1016/j.ydbio.2016.09.014. Epub 2016 Sep 14. Dev Biol. 2016. PMID: 27640087 Review.
-
Gastrointestinal tract modeling using organoids engineered with cellular and microbiota niches.Exp Mol Med. 2020 Feb;52(2):227-237. doi: 10.1038/s12276-020-0386-0. Epub 2020 Feb 26. Exp Mol Med. 2020. PMID: 32103122 Free PMC article. Review.
Cited by
-
Optimization of Vascularized Intestinal Organoid Model.Adv Healthc Mater. 2024 Dec;13(31):e2400977. doi: 10.1002/adhm.202400977. Epub 2024 Aug 1. Adv Healthc Mater. 2024. PMID: 39091070
-
Organoid, organ-on-a-chip and traditional Chinese medicine.Chin Med. 2025 Feb 12;20(1):22. doi: 10.1186/s13020-025-01071-8. Chin Med. 2025. PMID: 39940016 Free PMC article. Review.
-
Applications of human organoids in the personalized treatment for digestive diseases.Signal Transduct Target Ther. 2022 Sep 27;7(1):336. doi: 10.1038/s41392-022-01194-6. Signal Transduct Target Ther. 2022. PMID: 36167824 Free PMC article. Review.
-
Human Organoids for Predictive Toxicology Research and Drug Development.Front Genet. 2021 Nov 1;12:767621. doi: 10.3389/fgene.2021.767621. eCollection 2021. Front Genet. 2021. PMID: 34790228 Free PMC article. Review.
-
Preparation and Cultivation of Colonic and Small Intestinal Murine Organoids Including Analysis of Gene Expression and Organoid Viability.Bio Protoc. 2022 Jan 20;12(2):e4298. doi: 10.21769/BioProtoc.4298. eCollection 2022 Jan 20. Bio Protoc. 2022. PMID: 35127988 Free PMC article.
References
-
- Ben-David U, Siranosian B, Ha G, Tang H, Oren Y, Hinohara K, Strathdee CA, Dempster J, Lyons NJ, Burns R, Nag A, Kugener G, Cimini B, Tsvetkov P, Maruvka YE, O’Rourke R, Garrity A, Tubelli AA, Bandopadhayay P, Tsherniak A, Vazquez F, Wong B, Birger C, Ghandi M, Thorner AR, Bittker JA, Meyerson M, Getz G, Beroukhim R, Golub TR. Genetic and transcriptional evolution alters cancer cell line drug response. Nature 560: 325–330, 2018. doi:10.1038/s41586-018-0409-3. - DOI - PMC - PubMed
-
- Beyaz S, Mana MD, Roper J, Kedrin D, Saadatpour A, Hong S-J, Bauer-Rowe KE, Xifaras ME, Akkad A, Arias E, Pinello L, Katz Y, Shinagare S, Abu-Remaileh M, Mihaylova MM, Lamming DW, Dogum R, Guo G, Bell GW, Selig M, Nielsen GP, Gupta N, Ferrone CR, Deshpande V, Yuan G-C, Orkin SH, Sabatini DM, Yilmaz ÖH. High-fat diet enhances stemness and tumorigenicity of intestinal progenitors. Nature 531: 53–58, 2016. [Erratum in Nature 560: E26, 2018.] doi:10.1038/nature17173. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

