Inhibition of FPR2 impaired leukocytes recruitment and elicited non-resolving inflammation in acute heart failure
- PMID: 31216426
- PMCID: PMC6679729
- DOI: 10.1016/j.phrs.2019.104295
Inhibition of FPR2 impaired leukocytes recruitment and elicited non-resolving inflammation in acute heart failure
Abstract
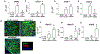
Lifestyle or age-related risk factors over-activate the inflammation that triggers acute heart failure (HF)-related mortality following myocardial infarction (MI). Post-MI activated leukocytes express formyl peptide receptor 2 (FPR2) that is essential for inflammation-resolution and in cardiac healing. However, the role of FPR2 in acute HF is incomplete and remain of interest. Here, we aimed to determine whether pharmacological inhibition of FPR2 perturb leukocyte trafficking in acute HF. Male C57BL/6 (8-12 weeks) mice were subjected to acute HF (MI-d1) using permanent coronary artery ligation that develops irreversible acute and chronic heart failure. FPR2 antagonist WRW4 (1 μg/kg/day) was subcutaneously injected 3 h post-MI maintaining saline-injected MI-controls. Leukocytes were quantitated using flow cytometry, and acute decompensated HF was confirmed using echocardiography and histology. FPR2 inhibition decreased the expression of FPR2 in the LV and spleen tissues. Administration of WRW4 inhibitor to mice primed immature and inactive neutrophils infiltration Ly6Gint and intensified the Ccl2 expression compared to MI-control in the infarcted LV post-MI. Leukocyte profiling revealed an overall decrease in monocytes (23.3 ± 2%) in WRW4-injected mice compared with MI-control (49.1 ± 2%) in infarcted LV. FPR2 inhibition increased F4/80+/Ly6Chi pro-inflammatory macrophages (14.8 ± 2%) compared with MI-control (10 ± 1%) with increased transcripts of pro-inflammatory markers TNF-α and IL-1β, and decreased Arg-1 expression in the infarcted LV compared to MI-controls is suggestive of the impaired acute inflammatory response. Inhibition of FPR2 using WRW4 also disturbed splenocardiac leukocytes recruitment by priming immature neutrophils leading to the onset of incomplete resolution signaling in acute decompensated HF post-MI.
Keywords: Formyl peptide receptor; Inflammation; Leukocytes; Myocardial infarction; Resolution of inflammation.
Published by Elsevier Ltd.
Conflict of interest statement
Figures



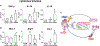
References
-
- Cui Y-H, Le Y, Gong W, Proost P, Van Damme J, Murphy WJ, Wang JM. Bacterial Lipopolysaccharide Selectively Up-Regulates the Function of the Chemotactic Peptide Receptor Formyl Peptide Receptor 2 in Murine Microglial Cells. The Journal of Immunology. 2002;168(1):434–442. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

