Caspases in Cell Death, Inflammation, and Disease
- PMID: 31216460
- PMCID: PMC6611727
- DOI: 10.1016/j.immuni.2019.05.020
Caspases in Cell Death, Inflammation, and Disease
Abstract
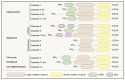
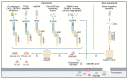
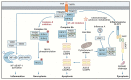
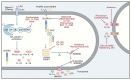
Caspases are an evolutionary conserved family of cysteine proteases that are centrally involved in cell death and inflammation responses. A wealth of foundational insight into the molecular mechanisms that control caspase activation has emerged in recent years. Important advancements include the identification of additional inflammasome platforms and pathways that regulate activation of inflammatory caspases; the discovery of gasdermin D as the effector of pyroptosis and interleukin (IL)-1 and IL-18 secretion; and the existence of substantial crosstalk between inflammatory and apoptotic initiator caspases. A better understanding of the mechanisms regulating caspase activation has supported initial efforts to modulate dysfunctional cell death and inflammation pathways in a suite of communicable, inflammatory, malignant, metabolic, and neurodegenerative diseases. Here, we review current understanding of caspase biology with a prime focus on the inflammatory caspases and outline important topics for future experimentation.
Keywords: apoptosis; caspase; gasdermin; inflammasome; interleukin; pyroptosis.
Copyright © 2019 Elsevier Inc. All rights reserved.
Conflict of interest statement
NVO and ML are employees of Janssen Pharmaceutica. The authors declare that they have no conflict of interest.
Figures
References
-
- Ablasser A, Chen ZJ. cGAS in action: Expanding roles in immunity and inflammation. Science. 2019;363 - PubMed
-
- Aubert DF, Xu H, Yang J, Shi X, Gao W, Li L, Bisaro F, Chen S, Valvano MA, Shao F. A Burkholderia Type VI Effector Deamidates Rho GTPases to Activate the Pyrin Inflammasome and Trigger Inflammation. Cell Host Microbe. 2016;19:664–674. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

