Gut Mycobiota in Immunity and Inflammatory Disease
- PMID: 31216461
- PMCID: PMC6585451
- DOI: 10.1016/j.immuni.2019.05.023
Gut Mycobiota in Immunity and Inflammatory Disease
Abstract
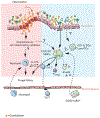
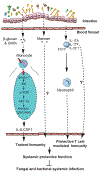
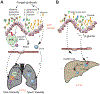
The mammalian intestine is colonized by a wealth of microorganisms-including bacteria, viruses, protozoa, and fungi-that are all integrated into a functional trans-kingdom community. Characterization of the composition of the fungal community-the mycobiota-has advanced further than the much-needed mechanistic studies. Recent findings have revealed roles for the gut mycobiota in the regulation of host immunity and in the development and progression of human diseases of inflammatory origin. We review these findings here while placing them in the context of the current understanding of the pathways and cellular networks that induce local and systemic immune responses to fungi in the gastrointestinal tract. We discuss gaps in knowledge and argue for the importance of considering bacteria-fungal interactions as we aim to define the roles of mycobiota in immune homeostasis and immune-associated pathologies.
Copyright © 2019 Elsevier Inc. All rights reserved.
Figures
References
-
- Allaire JM, Crowley SM, Law HT, Chang SY, Ko HJ, and Vallance BA (2019). The Intestinal Epithelium: Central Coordinator of Mucosal Immunity: (Trends in Immunology 39, 677-696, 2018). Trends in immunology 40, 174. - PubMed
-
- Arbuckle MR, McClain MT, Rubertone MV, Scofield RH, Dennis GJ, James JA, and Harley JB (2003). Development of autoantibodies before the clinical onset of systemic lupus erythematosus. The New England journal of medicine 349, 1526–1533. - PubMed
-
- Arrieta MC, Stiemsma LT, Dimitriu PA, Thorson L, Russell S, Yurist-Doutsch S, Kuzeljevic B, Gold MJ, Britton HM, Lefebvre DL, et al. (2015). Early infancy microbial and metabolic alterations affect risk of childhood asthma. Sci Transl Med 7, 307ra152. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

