Dynamic Modulation of Cortical Excitability during Visual Active Sensing
- PMID: 31216467
- PMCID: PMC6598687
- DOI: 10.1016/j.celrep.2019.05.072
Dynamic Modulation of Cortical Excitability during Visual Active Sensing
Abstract
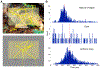
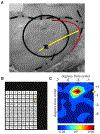
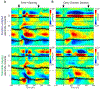
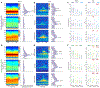
Visual physiology is traditionally investigated by presenting stimuli with gaze held constant. However, during active viewing of a scene, information is actively acquired using systematic patterns of fixations and saccades. Prior studies suggest that during such active viewing, both nonretinal, saccade-related signals and "extra-classical" receptive field inputs modulate visual processing. This study used a set of active viewing tasks that allowed us to compare visual responses with and without direct foveal input, thus isolating the contextual eye movement-related influences. Studying nonhuman primates, we find strong contextual modulation in primary visual cortex (V1): excitability and response amplification immediately after fixation onset, transiting to suppression leading up to the next saccade. Time-frequency decomposition suggests that this amplification and suppression cycle stems from a phase reset of ongoing neuronal oscillatory activity. The impact of saccade-related contextual modulation on stimulus processing makes active visual sensing fundamentally different from the more passive processes investigated in traditional paradigms.
Keywords: CSD; LFP; V1; active sensing; eye movements; local field potential; macaque; neuronal oscillations; saccadic modulation; vision.
Copyright © 2019 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
DECLARATION OF INTERESTS
The authors declare no competing interests.
Figures



References
-
- Burr DC, Morrone MC, and Ross J (1994). Selective suppression of the magnocellular visual pathway during saccadic eye movements. Nature 371, 511–513. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

