Arousal and sleep circuits
- PMID: 31216564
- PMCID: PMC6879642
- DOI: 10.1038/s41386-019-0444-2
Arousal and sleep circuits
Abstract
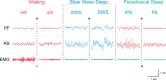
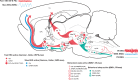
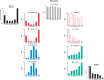
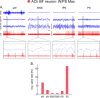
The principal neurons of the arousal and sleep circuits are comprised by glutamate and GABA neurons, which are distributed within the reticular core of the brain and, through local and distant projections and interactions, regulate cortical activity and behavior across wake-sleep states. These are in turn modulated by the neuromodulatory systems that are comprised by acetylcholine, noradrenaline, dopamine, serotonin, histamine, orexin (hypocretin), and melanin-concentrating hormone (MCH) neurons. Glutamate and GABA neurons are heterogeneous in their profiles of discharge, forming distinct functional cell types by selective or maximal discharge during (1) waking and paradoxical (REM) sleep, (2) during slow wave sleep, (3) during waking, or (4) during paradoxical (REM) sleep. The neuromodulatory systems are each homogeneous in their profile of discharge, the majority discharging maximally during waking and paradoxical sleep or during waking. Only MCH neurons discharge maximally during sleep. They each exert their modulatory influence upon other neurons through excitatory and inhibitory receptors thus effecting a concerted differential change in the functionally different cell groups. Both arousal and sleep circuit neurons are homeostatically regulated as a function of their activity in part through changes in receptors. The major pharmacological agents used for the treatment of wake and sleep disorders act upon GABA and neuromodulatory transmission.
Conflict of interest statement
The author declares no competing interests.
Figures
References
-
- Maloney KJ, Cape EG, Gotman J, Jones BE. High-frequency gamma electroencephalogram activity in association with sleep-wake states and spontaneous behaviors in the rat. Neuroscience. 1997;76:541–55. - PubMed
-
- Moruzzi G, Magoun HW. Brain stem reticular formation and activation of the EEG. Electroencephalogr Clin Neurophysiol. 1949;1:455–73. - PubMed
-
- Lindsley DB, Schreiner LH, Knowles WB, Magoun HW. Behavioral and EEG changes following chronic brain stem lesions. Electroencephalogr Clin Neurophysiol. 1950;2:483–98. - PubMed
-
- Plum F, Posner JB. The diagnosis of stupor and coma. Philadelphia: Davis; 1980.
-
- Parvizi J, Damasio AR. Neuroanatomical correlates of brainstem coma. Brain. 2003;126:1524–36. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

