Role of Key Micronutrients from Nutrigenetic and Nutrigenomic Perspectives in Cancer Prevention
- PMID: 31216637
- PMCID: PMC6630934
- DOI: 10.3390/medicina55060283
Role of Key Micronutrients from Nutrigenetic and Nutrigenomic Perspectives in Cancer Prevention
Abstract
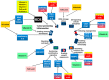
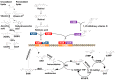
Regarding cancer as a genetic multi-factorial disease, a number of aspects need to be investigated and analyzed in terms of cancer's predisposition, development and prognosis. One of these multi-dimensional factors, which has gained increased attention in the oncological field due to its unelucidated role in risk assessment for cancer, is diet. Moreover, as studies advance, a clearer connection between diet and the molecular alteration of patients is becoming identifiable and quantifiable, thereby replacing the old general view associating specific phenotypical changes with the differential intake of nutrients. Respectively, there are two major fields concentrated on the interrelation between genome and diet: nutrigenetics and nutrigenomics. Nutrigenetics studies the effects of nutrition at the gene level, whereas nutrigenomics studies the effect of nutrients on genome and transcriptome patterns. By precisely evaluating the interaction between the genomic profile of patients and their nutrient intake, it is possible to envision a concept of personalized medicine encompassing nutrition and health care. The list of nutrients that could have an inhibitory effect on cancer development is quite extensive, with evidence in the scientific literature. The administration of these nutrients showed significant results in vitro and in vivo regarding cancer inhibition, although more studies regarding administration in effective doses in actual patients need to be done.
Keywords: cancer; chemoprevention; nutrigenetics; nutrigenomics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
New criteria for supplementation of selected micronutrients in the era of nutrigenetics and nutrigenomics.Int J Food Sci Nutr. 2014 Aug;65(5):529-38. doi: 10.3109/09637486.2014.898258. Epub 2014 Mar 13. Int J Food Sci Nutr. 2014. PMID: 24625102 Review.
-
Micronutritional approaches to periodontal therapy.J Clin Periodontol. 2011 Mar;38 Suppl 11:142-58. doi: 10.1111/j.1600-051X.2010.01663.x. J Clin Periodontol. 2011. PMID: 21323711 Review.
-
Nutrigenetics and nutrigenomics: viewpoints on the current status and applications in nutrition research and practice.J Nutrigenet Nutrigenomics. 2011;4(2):69-89. doi: 10.1159/000327772. Epub 2011 May 28. J Nutrigenet Nutrigenomics. 2011. PMID: 21625170 Free PMC article. Review.
-
TRIENNIAL LACTATION SYMPOSIUM: Nutrigenomics in dairy cows: Nutrients, transcription factors, and techniques.J Anim Sci. 2015 Dec;93(12):5531-53. doi: 10.2527/jas.2015-9192. J Anim Sci. 2015. PMID: 26641164
-
Nutrigenomics: SNPs correlated to minerals' deficiencies.Clin Ter. 2023 Nov-Dec;174(Suppl 2(6)):193-199. doi: 10.7417/CT.2023.2487. Clin Ter. 2023. PMID: 37994764 Review.
Cited by
-
A review of the Mediterranean diet and nutritional genomics in relation to cancer in women.J Prev Med Hyg. 2022 Oct 17;63(2 Suppl 3):E81-E86. doi: 10.15167/2421-4248/jpmh2022.63.2S3.2750. eCollection 2022 Jun. J Prev Med Hyg. 2022. PMID: 36479503 Free PMC article. Review.
-
A Comprehensive Review on MAPK: A Promising Therapeutic Target in Cancer.Cancers (Basel). 2019 Oct 22;11(10):1618. doi: 10.3390/cancers11101618. Cancers (Basel). 2019. PMID: 31652660 Free PMC article. Review.
-
Role of Antioxidant Vitamins and Other Micronutrients on Regulations of Specific Genes and Signaling Pathways in the Prevention and Treatment of Cancer.Int J Mol Sci. 2023 Mar 23;24(7):6092. doi: 10.3390/ijms24076092. Int J Mol Sci. 2023. PMID: 37047063 Free PMC article. Review.
-
Nutrigenomics and microbiome shaping the future of personalized medicine: a review article.J Genet Eng Biotechnol. 2023 Nov 22;21(1):134. doi: 10.1186/s43141-023-00599-2. J Genet Eng Biotechnol. 2023. PMID: 37993702 Free PMC article. Review.
-
Clinical efficacy and safety of oral and intravenous vitamin C use in patients with malignant diseases.J Cancer Res Clin Oncol. 2021 Oct;147(10):3025-3042. doi: 10.1007/s00432-021-03759-4. Epub 2021 Aug 17. J Cancer Res Clin Oncol. 2021. PMID: 34402972 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

