Novel Stenotic Microchannels to Study Thrombus Formation in Shear Gradients: Influence of Shear Forces and Human Platelet-Related Factors
- PMID: 31216638
- PMCID: PMC6627598
- DOI: 10.3390/ijms20122967
Novel Stenotic Microchannels to Study Thrombus Formation in Shear Gradients: Influence of Shear Forces and Human Platelet-Related Factors
Abstract
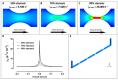
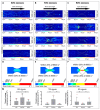
Thrombus formation in hemostasis or thrombotic disease is initiated by the rapid adhesion, activation, and aggregation of circulating platelets in flowing blood. At arterial or pathological shear rates, for example due to vascular stenosis or circulatory support devices, platelets may be exposed to highly pulsatile blood flow, while even under constant flow platelets are exposed to pulsation due to thrombus growth or changes in vessel geometry. The aim of this study is to investigate platelet thrombus formation dynamics within flow conditions consisting of either constant or variable shear. Human platelets in anticoagulated whole blood were exposed ex vivo to collagen type I-coated microchannels subjected to constant shear in straight channels or variable shear gradients using different stenosis geometries (50%, 70%, and 90% by area). Base wall shears between 1800 and 6600 s-1, and peak wall shears of 3700 to 29,000 s-1 within stenoses were investigated, representing arterial-pathological shear conditions. Computational flow-field simulations and stenosis platelet thrombi total volume, average volume, and surface coverage were analysed. Interestingly, shear gradients dramatically changed platelet thrombi formation compared to constant base shear alone. Such shear gradients extended the range of shear at which thrombi were formed, that is, platelets became hyperthrombotic within shear gradients. Furthermore, individual healthy donors displayed quantifiable differences in extent/formation of thrombi within shear gradients, with implications for future development and testing of antiplatelet agents. In conclusion, here, we demonstrate a specific contribution of blood flow shear gradients to thrombus formation, and provide a novel platform for platelet functional testing under shear conditions.
Keywords: platelets; shear gradients; stenosis; thrombosis.
Conflict of interest statement
The authors declare no conflicts of interest. Funders played no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures

Similar articles
-
Effects of upstream shear forces on priming of platelets for downstream adhesion and activation.Acta Biomater. 2018 Jun;73:228-235. doi: 10.1016/j.actbio.2018.04.002. Epub 2018 Apr 11. Acta Biomater. 2018. PMID: 29654993 Free PMC article.
-
Downstream platelet adhesion and activation under highly elevated upstream shear forces.Acta Biomater. 2019 Jun;91:135-143. doi: 10.1016/j.actbio.2019.04.028. Epub 2019 Apr 17. Acta Biomater. 2019. PMID: 31004847 Free PMC article.
-
Effects of different shear rates on the attachment and detachment of platelet thrombi.Mol Med Rep. 2016 Mar;13(3):2447-56. doi: 10.3892/mmr.2016.4825. Epub 2016 Jan 28. Mol Med Rep. 2016. PMID: 26847168 Free PMC article.
-
VWF attributes--impact on thrombus formation.Thromb Res. 2008;122 Suppl 4:S9-13. doi: 10.1016/S0049-3848(08)70028-8. Thromb Res. 2008. PMID: 18929523 Review.
-
Shear-Dependent Platelet Aggregation: Mechanisms and Therapeutic Opportunities.Front Cardiovasc Med. 2019 Sep 20;6:141. doi: 10.3389/fcvm.2019.00141. eCollection 2019. Front Cardiovasc Med. 2019. PMID: 31620451 Free PMC article. Review.
Cited by
-
Label-free multimodal quantitative imaging flow assay for intrathrombus formation in vitro.Biophys J. 2021 Mar 2;120(5):791-804. doi: 10.1016/j.bpj.2021.01.015. Epub 2021 Jan 26. Biophys J. 2021. PMID: 33513336 Free PMC article.
-
Platelet Adhesion and Thrombus Formation in Microchannels: The Effect of Assay-Dependent Variables.Int J Mol Sci. 2020 Jan 23;21(3):750. doi: 10.3390/ijms21030750. Int J Mol Sci. 2020. PMID: 31979370 Free PMC article.
-
Thrombosis and Hemodynamics: external and intrathrombus gradients.Curr Opin Biomed Eng. 2021 Sep;19:100316. doi: 10.1016/j.cobme.2021.100316. Epub 2021 Jun 26. Curr Opin Biomed Eng. 2021. PMID: 34693101 Free PMC article.
-
Imaging Platelet Processes and Function-Current and Emerging Approaches for Imaging in vitro and in vivo.Front Immunol. 2020 Jan 31;11:78. doi: 10.3389/fimmu.2020.00078. eCollection 2020. Front Immunol. 2020. PMID: 32082328 Free PMC article. Review.
-
Blood Flow Velocimetry in a Microchannel During Coagulation Using Particle Image Velocimetry and Wavelet-Based Optical Flow Velocimetry.J Biomech Eng. 2021 Sep 1;143(9):091004. doi: 10.1115/1.4050647. J Biomech Eng. 2021. PMID: 33764427 Free PMC article.
References
-
- Al-Tamimi M., Tan C.W., Qiao J., Pennings G.J., Javadzadegan A., Yong A.S.C., Arthur J.F., Davis A.K., Jing J., Mu F.-T., et al. Pathological shear triggers shedding of vascular receptors: A novel mechanism for downregulation of platelet glycoprotein (GP)VI in stenosed coronary vessels. Blood. 2012;119:4311–4320. doi: 10.1182/blood-2011-10-386607. - DOI - PubMed
-
- Gardiner E.E., Andrews R.K. Platelet adhesion. In: Gresele P., Lopez J.A., Kleiman N.S., Page C.P., editors. Platelets in Thrombotic and Non-Thrombotic Disorders. Springer; Berlin/Heidelberg, Germany: 2017. pp. 309–319.
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

