Chloroplast Outer Membrane β-Barrel Proteins Use Components of the General Import Apparatus
- PMID: 31217220
- PMCID: PMC6713306
- DOI: 10.1105/tpc.19.00001
Chloroplast Outer Membrane β-Barrel Proteins Use Components of the General Import Apparatus
Abstract
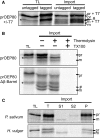
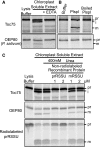
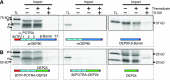
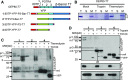
Chloroplasts evolved from a cyanobacterial endosymbiont that resided within a eukaryotic cell. Due to their prokaryotic heritage, chloroplast outer membranes contain transmembrane β-barrel proteins. While most chloroplast proteins use N-terminal transit peptides to enter the chloroplasts through the translocons at the outer and inner chloroplast envelope membranes (TOC/TIC), only one β-barrel protein, Toc75, has been shown to use this pathway. The route other β-barrel proteins use has remained unresolved. Here we use in vitro pea (Pisum sativum) chloroplast import assays and transient expression in Nicotiana benthamiana to address this. We show that a paralog of Toc75, outer envelope protein 80 kD (OEP80), also uses a transit peptide but has a distinct envelope sorting signal. Our results additionally indicate that β-barrels that do not use transit peptides also enter the chloroplast using components of the general import pathway.
© 2019 American Society of Plant Biologists. All rights reserved.
Figures

Comment in
-
Barreling Down the Chloroplast Highway: Protein Sorting of Outer-Membrane β-Barrel Proteins.Plant Cell. 2019 Aug;31(8):1679-1680. doi: 10.1105/tpc.19.00452. Epub 2019 Jun 19. Plant Cell. 2019. PMID: 31217221 Free PMC article. No abstract available.
References
-
- Baldwin A.J., Inoue K. (2006). The most C-terminal tri-glycine segment within the polyglycine stretch of the pea Toc75 transit peptide plays a critical role for targeting the protein to the chloroplast outer envelope membrane. FEBS J. 273: 1547–1555. - PubMed
-
- Bölter B. (2018). En route into chloroplasts: Preproteins’ way home. Photosynth. Res. 138: 263–275. - PubMed

