Human Cytomegalovirus Compromises Development of Cerebral Organoids
- PMID: 31217239
- PMCID: PMC6694831
- DOI: 10.1128/JVI.00957-19
Human Cytomegalovirus Compromises Development of Cerebral Organoids
Abstract
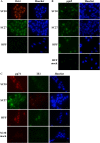
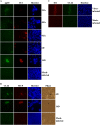
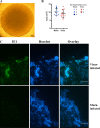
Congenital human cytomegalovirus (HCMV) infection causes a broad spectrum of central and peripheral nervous system disorders, ranging from microcephaly to hearing loss. These ramifications mandate the study of virus-host interactions in neural cells. Neural progenitor cells are permissive for lytic infection. We infected two induced pluripotent stem cell (iPSC) lines and found these more primitive cells to be susceptible to infection but not permissive. Differentiation of infected iPSCs induced de novo expression of viral antigens. iPSCs can be cultured in three dimensions to generate cerebral organoids, closely mimicking in vivo development. Mock- or HCMV-infected iPSCs were subjected to a cerebral organoid generation protocol. HCMV IE1 protein was detected in virus-infected organoids at 52 days postinfection. Absent a significant effect on organoid size, infection induced regions of necrosis and the presence of large vacuoles and cysts. Perhaps more in parallel with the subtler manifestations of HCMV-induced birth defects, infection dramatically altered neurological development of organoids, decreasing the number of developing and fully formed cortical structure sites, with associated changes in the architectural organization and depth of lamination within these structures, and manifesting aberrant expression of the neural marker β-tubulin III. Our observations parallel published descriptions of infected clinical samples, which often contain only sparse antigen-positive foci yet display areas of focal necrosis and cellular loss, delayed maturation, and abnormal cortical lamination. The parallels between pathologies present in clinical specimens and the highly tractable three-dimensional (3D) organoid system demonstrate the utility of this system in modeling host-virus interactions and HCMV-induced birth defects.IMPORTANCE Human cytomegalovirus (HCMV) is a leading cause of central nervous system birth defects, ranging from microcephaly to hearing impairment. Recent literature has provided descriptions of delayed and abnormal maturation of developing cortical tissue in infected clinical specimens. We have found that infected induced pluripotent stem cells can be differentiated into three-dimensional, viral protein-expressing cerebral organoids. Virus-infected organoids displayed dramatic alterations in development compared to those of mock-infected controls. Development in these organoids closely paralleled observations in HCMV-infected clinical samples. Infection induced regions of necrosis, the presence of larger vacuoles and cysts, changes in the architectural organization of cortical structures, aberrant expression of the neural marker β-tubulin III, and an overall reduction in numbers of cortical structure sites. We found clear parallels between the pathologies of clinical specimens and virus-infected organoids, demonstrating the utility of this highly tractable system for future investigations of HCMV-induced birth defects.
Keywords: cerebral organoid; cortical development; human cytomegalovirus; induced pluripotent stem cells; neural marker expression.
Copyright © 2019 American Society for Microbiology.
Figures



Similar articles
-
Human Cytomegalovirus Disruption of Calcium Signaling in Neural Progenitor Cells and Organoids.J Virol. 2019 Aug 13;93(17):e00954-19. doi: 10.1128/JVI.00954-19. Print 2019 Sep 1. J Virol. 2019. PMID: 31217241 Free PMC article.
-
Nitric Oxide Attenuates Human Cytomegalovirus Infection yet Disrupts Neural Cell Differentiation and Tissue Organization.J Virol. 2022 Jul 27;96(14):e0012622. doi: 10.1128/jvi.00126-22. Epub 2022 Jul 7. J Virol. 2022. PMID: 35862705 Free PMC article.
-
HCMV Infection Reduces Nidogen-1 Expression, Contributing to Impaired Neural Rosette Development in Brain Organoids.J Virol. 2023 May 31;97(5):e0171822. doi: 10.1128/jvi.01718-22. Epub 2023 May 1. J Virol. 2023. PMID: 37125912 Free PMC article.
-
Brain Organoids: Expanding Our Understanding of Human Development and Disease.Results Probl Cell Differ. 2018;66:183-206. doi: 10.1007/978-3-319-93485-3_8. Results Probl Cell Differ. 2018. PMID: 30209660 Review.
-
The Emergence of Stem Cell-Based Brain Organoids: Trends and Challenges.Bioessays. 2019 Aug;41(8):e1900011. doi: 10.1002/bies.201900011. Epub 2019 Jul 5. Bioessays. 2019. PMID: 31274205 Review.
Cited by
-
Harness Organoid Models for Virological Studies in Animals: A Cross-Species Perspective.Front Microbiol. 2021 Sep 16;12:725074. doi: 10.3389/fmicb.2021.725074. eCollection 2021. Front Microbiol. 2021. PMID: 34603253 Free PMC article. Review.
-
Modeling SARS-CoV-2 infection in individuals with opioid use disorder with brain organoids.J Tissue Eng. 2021 Feb 26;12:2041731420985299. doi: 10.1177/2041731420985299. eCollection 2021 Jan-Dec. J Tissue Eng. 2021. PMID: 33738089 Free PMC article. Review.
-
Parechovirus infection in human brain organoids: host innate inflammatory response and not neuro-infectivity correlates to neurologic disease.Nat Commun. 2024 Mar 21;15(1):2532. doi: 10.1038/s41467-024-46634-9. Nat Commun. 2024. PMID: 38514653 Free PMC article.
-
Next-Generation Human Cerebral Organoids as Powerful Tools To Advance NeuroHIV Research.mBio. 2021 Aug 31;12(4):e0068021. doi: 10.1128/mBio.00680-21. Epub 2021 Jul 13. mBio. 2021. PMID: 34253056 Free PMC article. Review.
-
Advancing insights into virus-induced neurodevelopmental disorders through human brain organoid modelling.Expert Rev Mol Med. 2024 Nov 26;27:e1. doi: 10.1017/erm.2024.35. Expert Rev Mol Med. 2024. PMID: 39587735 Free PMC article. Review.
References
-
- Britt W, Alford C. 1996. Cytomegalovirus, p 2493–2523. In Fields BN, Knipe DM, Howley PM (ed), Fields virology, 3rd ed Lippincott-Raven Publishers, Philadelphia, PA.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

