The Novel Avian Leukosis Virus Subgroup K Shares Its Cellular Receptor with Subgroup A
- PMID: 31217247
- PMCID: PMC6694804
- DOI: 10.1128/JVI.00580-19
The Novel Avian Leukosis Virus Subgroup K Shares Its Cellular Receptor with Subgroup A
Abstract
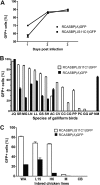
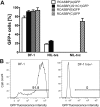
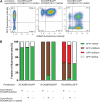
Avian leukosis virus subgroup K (ALV-K) is composed of newly emerging isolates, which, in sequence analyses, cluster separately from the well-characterized subgroups A, B, C, D, E, and J. However, it remains unclear whether ALV-K represents an independent ALV subgroup with regard to receptor usage, host range, and superinfection interference. In the present study, we examined the host range of the Chinese infectious isolate JS11C1, an ALV-K prototype, and we found substantial overlap of species that were either resistant or susceptible to ALV-A and JS11C1. Ectopic expression of the chicken tva gene in mammalian cells conferred susceptibility to JS11C1, while genetic ablation of the tva gene rendered chicken DF-1 cells resistant to infection by JS11C1. Thus, tva expression is both sufficient and necessary for JS11C1 entry. Receptor sharing was also manifested in superinfection interference, with preinfection of cells with ALV-A, but not ALV-B or ALV-J, blocking subsequent JS11C1 infection. Finally, direct binding of JS11C1 and Tva was demonstrated by preincubation of the virus with soluble Tva, which substantially decreased viral infectivity in susceptible chicken cells. Collectively, these findings indicate that JS11C1 represents a new and bona fide ALV subgroup that utilizes Tva for cell entry and binds to a site other than that for ALV-A.IMPORTANCE ALV consists of several subgroups that are particularly characterized by their receptor usage, which subsequently dictates the host range and tropism of the virus. A few newly emerging and highly pathogenic Chinese ALV strains have recently been suggested to be an independent subgroup, ALV-K, based solely on their genomic sequences. Here, we performed a series of experiments with the ALV-K strain JS11C1, which showed its dependence on the Tva cell surface receptor. Due to the sharing of this receptor with ALV-A, both subgroups were able to interfere with superinfection. Because ALV-K could become an important pathogen and a significant threat to the poultry industry in Asia, the identification of a specific receptor could help in the breeding of resistant chicken lines with receptor variants with decreased susceptibility to the virus.
Keywords: Tva; avian leukosis virus K; host range; resistance/susceptibility to retrovirus; retrovirus receptor; superinfection interference.
Copyright © 2019 American Society for Microbiology.
Figures

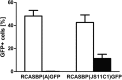
Similar articles
-
Insertion/deletion mutations within tva receptor gene confer chicken resistance to infection by avian leukosis virus subgroups A and K.Poult Sci. 2025 Apr;104(4):104949. doi: 10.1016/j.psj.2025.104949. Epub 2025 Feb 26. Poult Sci. 2025. PMID: 40048979 Free PMC article.
-
Single nucleotide polymorphism variants within tva and tvb receptor genes in Chinese chickens.Poult Sci. 2014 Oct;93(10):2482-9. doi: 10.3382/ps.2014-04077. Epub 2014 Aug 14. Poult Sci. 2014. PMID: 25125563
-
Single Amino Acid Residue W33 of tva Receptor Is Critical for Viral Entry and High-Affinity Binding of Avian Leukosis Virus Subgroup K.Viruses. 2025 May 15;17(5):709. doi: 10.3390/v17050709. Viruses. 2025. PMID: 40431720 Free PMC article.
-
Current knowledge on the epidemiology and prevention of Avian leukosis virus in China.Poult Sci. 2024 Sep;103(9):104009. doi: 10.1016/j.psj.2024.104009. Epub 2024 Jun 25. Poult Sci. 2024. PMID: 39002365 Free PMC article. Review.
-
Avian Leukosis: Will We Be Able to Get Rid of It?Animals (Basel). 2023 Jul 19;13(14):2358. doi: 10.3390/ani13142358. Animals (Basel). 2023. PMID: 37508135 Free PMC article. Review.
Cited by
-
Advances on genetic and genomic studies of ALV resistance.J Anim Sci Biotechnol. 2022 Oct 11;13(1):123. doi: 10.1186/s40104-022-00769-1. J Anim Sci Biotechnol. 2022. PMID: 36217167 Free PMC article. Review.
-
Knock-Out of Retrovirus Receptor Gene Tva in the Chicken Confers Resistance to Avian Leukosis Virus Subgroups A and K and Affects Cobalamin (Vitamin B12)-Dependent Level of Methylmalonic Acid.Viruses. 2021 Dec 14;13(12):2504. doi: 10.3390/v13122504. Viruses. 2021. PMID: 34960774 Free PMC article.
-
Reverse Engineering Provides Insights on the Evolution of Subgroups A to E Avian Sarcoma and Leukosis Virus Receptor Specificity.Viruses. 2019 May 30;11(6):497. doi: 10.3390/v11060497. Viruses. 2019. PMID: 31151254 Free PMC article. Review.
-
Insertion/deletion mutations within tva receptor gene confer chicken resistance to infection by avian leukosis virus subgroups A and K.Poult Sci. 2025 Apr;104(4):104949. doi: 10.1016/j.psj.2025.104949. Epub 2025 Feb 26. Poult Sci. 2025. PMID: 40048979 Free PMC article.
-
The Current View of Retroviruses as Seen from the Shoulders of a Giant.Viruses. 2019 Sep 5;11(9):828. doi: 10.3390/v11090828. Viruses. 2019. PMID: 31491994 Free PMC article.
References
-
- Weiss RA. 1992. Cellular receptors and viral glycoproteins involved in retrovirus entry, p 1–108. In Levy JA. (ed), Retroviridae, vol 2 Plenum Press, New York, NY.
-
- Elleder D, Stepanets V, Melder DC, Šenigl F, Geryk J, Pajer P, Plachý J, Hejnar J, Svoboda J, Federspiel MJ. 2005. The receptor for the subgroup C avian sarcoma and leukosis viruses, Tvc, is related to mammalian butyrophilins, members of the immunoglobulin superfamily. J Virol 79:10408–10419. doi:10.1128/JVI.79.16.10408-10419.2005. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

