TRIOBP-5 sculpts stereocilia rootlets and stiffens supporting cells enabling hearing
- PMID: 31217345
- PMCID: PMC6629139
- DOI: 10.1172/jci.insight.128561
TRIOBP-5 sculpts stereocilia rootlets and stiffens supporting cells enabling hearing
Abstract
TRIOBP remodels the cytoskeleton by forming unusually dense F-actin bundles and is implicated in human cancer, schizophrenia, and deafness. Mutations ablating human and mouse TRIOBP-4 and TRIOBP-5 isoforms are associated with profound deafness, as inner ear mechanosensory hair cells degenerate after stereocilia rootlets fail to develop. However, the mechanisms regulating formation of stereocilia rootlets by each TRIOBP isoform remain unknown. Using 3 new Triobp mouse models, we report that TRIOBP-5 is essential for thickening bundles of F-actin in rootlets, establishing their mature dimensions and for stiffening supporting cells of the auditory sensory epithelium. The coiled-coil domains of this isoform are required for reinforcement and maintenance of stereocilia rootlets. A loss of TRIOBP-5 in mouse results in dysmorphic rootlets that are abnormally thin in the cuticular plate but have increased widths and lengths within stereocilia cores, and causes progressive deafness recapitulating the human phenotype. Our study extends the current understanding of TRIOBP isoform-specific functions necessary for life-long hearing, with implications for insight into other TRIOBPopathies.
Keywords: Cytoskeleton; Neuroscience.
Conflict of interest statement
Figures

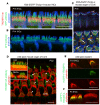
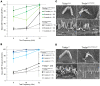
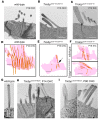
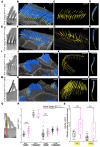



References
-
- Bao J, Wang S, Gunther LK, Kitajiri S, Li C, Sakamoto T. The actin-bundling protein TRIOBP-4 and -5 promotes the motility of pancreatic cancer cells. Cancer Lett. 2015;356(2 Pt B):367–373. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

