Antisense oligonucleotide treatment ameliorates IFN-γ-induced proteinuria in APOL1-transgenic mice
- PMID: 31217349
- PMCID: PMC6629101
- DOI: 10.1172/jci.insight.126124
Antisense oligonucleotide treatment ameliorates IFN-γ-induced proteinuria in APOL1-transgenic mice
Abstract
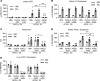
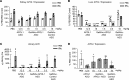
African Americans develop end-stage renal disease at a higher rate compared with European Americans due to 2 polymorphisms (G1 and G2 risk variants) in the apolipoprotein L1 (APOL1) gene common in people of African ancestry. Although this compelling genetic evidence provides an exciting opportunity for personalized medicine in chronic kidney disease, drug discovery efforts have been greatly hindered by the fact that APOL1 expression is lacking in rodents. Here, we describe a potentially novel physiologically relevant genomic mouse model of APOL1-associated renal disease that expresses human APOL1 from the endogenous human promoter, resulting in expression in similar tissues and at similar relative levels as humans. While naive APOL1-transgenic mice did not exhibit a renal disease phenotype, administration of IFN-γ was sufficient to robustly induce proteinuria only in APOL1 G1 mice, despite inducing kidney APOL1 expression in both G0 and G1 mice, serving as a clinically relevant "second hit." Treatment of APOL1 G1 mice with IONIS-APOL1Rx, an antisense oligonucleotide (ASO) targeting APOL1 mRNA, prior to IFN-γ challenge robustly and dose-dependently inhibited kidney and liver APOL1 expression and protected against IFN-γ-induced proteinuria, indicating that the disease-relevant cell types are sensitive to ASO treatment. Therefore, IONIS-APOL1Rx may be an effective therapeutic for APOL1 nephropathies and warrants further development.
Keywords: Drug therapy; Genetic diseases; Mouse models; Nephrology; Therapeutics.
Conflict of interest statement
Figures






References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

