Sniffer cells for the detection of neural Angiotensin II in vitro
- PMID: 31217439
- PMCID: PMC6584535
- DOI: 10.1038/s41598-019-45262-4
Sniffer cells for the detection of neural Angiotensin II in vitro
Abstract
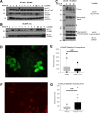
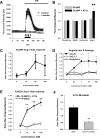
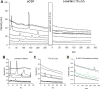
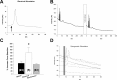
Neuropeptide release in the brain has traditionally been difficult to observe. Existing methods lack temporal and spatial resolution that is consistent with the function and size of neurons. We use cultured "sniffer cells" to improve the temporal and spatial resolution of observing neuropeptide release. Sniffer cells were created by stably transfecting Chinese Hamster Ovary (CHO) cells with plasmids encoding the rat angiotensin type 1a receptor and a genetically encoded Ca2+ sensor. Isolated, cultured sniffer cells showed dose-dependent increases in fluorescence in response to exogenously applied angiotensin II and III, but not other common neurotransmitters. Sniffer cells placed on the median preoptic nucleus (a presumptive site of angiotensin release) displayed spontaneous activity and evoked responses to either electrical or optogenetic stimulation of the subfornical organ. Stable sniffer cell lines could be a viable method for detecting neuropeptide release in vitro, while still being able to distinguish differences in neuropeptide concentration.
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Spontaneous and evoked angiotensin II sniffer cell activity in the lamina terminalis in vitro.Am J Physiol Regul Integr Comp Physiol. 2024 Nov 1;327(5):R486-R496. doi: 10.1152/ajpregu.00227.2023. Epub 2024 Aug 12. Am J Physiol Regul Integr Comp Physiol. 2024. PMID: 39133776 Free PMC article.
-
Detection of activity-dependent vasopressin release from neuronal dendrites and axon terminals using sniffer cells.J Neurophysiol. 2018 Sep 1;120(3):1386-1396. doi: 10.1152/jn.00467.2017. Epub 2018 Jul 5. J Neurophysiol. 2018. PMID: 29975164
-
Ontogeny of angiotensin II type 1 receptor mRNAs in fetal and neonatal rat brain.J Comp Neurol. 2001 Nov 12;440(2):192-203. doi: 10.1002/cne.1379. J Comp Neurol. 2001. PMID: 11745617
-
Detection of angiotensin II type 1 receptor ligands by a cell-based assay.Anal Bioanal Chem. 2009 Nov;395(6):1937-40. doi: 10.1007/s00216-009-3074-4. Anal Bioanal Chem. 2009. PMID: 19760406
-
Expression of angiotensin type-1 (AT1) and type-2 (AT2) receptor mRNAs in the adult rat brain: a functional neuroanatomical review.Front Neuroendocrinol. 1997 Oct;18(4):383-439. doi: 10.1006/frne.1997.0155. Front Neuroendocrinol. 1997. PMID: 9344632 Review.
Cited by
-
Role of angiotensin II in chronic intermittent hypoxia-induced hypertension and cognitive decline.Am J Physiol Regul Integr Comp Physiol. 2021 Apr 1;320(4):R519-R525. doi: 10.1152/ajpregu.00222.2020. Epub 2021 Feb 17. Am J Physiol Regul Integr Comp Physiol. 2021. PMID: 33595364 Free PMC article. Review.
-
Spontaneous and evoked angiotensin II sniffer cell activity in the lamina terminalis in vitro.Am J Physiol Regul Integr Comp Physiol. 2024 Nov 1;327(5):R486-R496. doi: 10.1152/ajpregu.00227.2023. Epub 2024 Aug 12. Am J Physiol Regul Integr Comp Physiol. 2024. PMID: 39133776 Free PMC article.
-
Sodium Intake and Disease: Another Relationship to Consider.Nutrients. 2023 Jan 19;15(3):535. doi: 10.3390/nu15030535. Nutrients. 2023. PMID: 36771242 Free PMC article. Review.
References
-
- Paul M, Bader M, Steckelings UM, Voigtlander T, Ganten D. The renin-angiotensin system in the brain. Localization and functional significance. Arzneimittel-Forschung. 1993;43:207–213. - PubMed
-
- Bai D, Renaud LP. ANG II AT1 receptors induce depolarization and inward current in rat median preoptic neurons in vitro. The American journal of physiology. 1998;275:R632–639. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

