Role of Cav-1 in HIV-1 Tat-Induced Dysfunction of Tight Junctions and A β-Transferring Proteins
- PMID: 31217837
- PMCID: PMC6537002
- DOI: 10.1155/2019/3403206
Role of Cav-1 in HIV-1 Tat-Induced Dysfunction of Tight Junctions and A β-Transferring Proteins
Abstract
Objective: To evaluate the role of caveolin-1 (Cav-1) in HIV-1 Tat-induced dysfunction of tight junction and amyloid β-peptide- (Aβ-) transferring proteins.
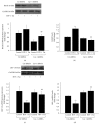
Methods: A Cav-1 shRNA interference target sequence was cloned into the lentiviral vector pHBLV-U6-Scramble-ZsGreen-Puro and verified by double enzyme digestion and DNA sequencing. Human cerebral microvascular endothelium (HBEC-5i) cells were transduced with viral particles made in 293T cells by transfection with lentiviral packaging plasmids. HBEC-5i cells transduced with Cav-1 shRNA or Ctr shRNA were exposed to HIV-1 Tat for 24 h, and the protein and mRNA levels of the tight junction protein occludin, Aβ-transferring protein, receptor for advanced glycation end products (RAGE), low-density lipoprotein receptor-related protein- (LRP-) 1, and RhoA were evaluated with Western blot and real-time reverse transcription polymerase chain reaction (qRT-PCR) assays, respectively.
Results: After sequencing, an RNA interference recombinant lentivirus expressing a vector targeting Cav-1 was successfully established. The recombined lentiviral particles were made by using 293T cells to package the recombined lentiviral vector. A stable monoclonal cell line with strong GFP expression was acquired with a Cav-1 knockdown rate of 85.7%. The occludin protein and mRNA levels in the Ctr shRNA group were decreased with HIV-1 Tat exposure but were upregulated in the Cav-1 shRNA group. The HIV-1 Tat-induced alterations of RAGE and LRP-1 protein and mRNA levels in the Ctr shRNA group were attenuated in the Cav-1 shRNA group. The RhoA protein levels in the Ctr shRNA group were upregulated by HIV-1 Tat exposure but were downregulated in the Cav-1 shRNA group.
Conclusion: These results show that HIV-1 Tat-induced downregulation of occludin and LRP-1 and upregulation of RAGE and RhoA may result in the accumulation of Aβ in the brain. Silencing the Cav-1 gene with shRNA plays a key role in the protection against HIV-1 Tat-induced dysfunction of the blood-brain barrier and Aβ accumulation.
Figures




Similar articles
-
Rho-kinase inhibitor hydroxyfasudil protects against HIV-1 Tat-induced dysfunction of tight junction and neprilysin/Aβ transfer receptor expression in mouse brain microvessels.Mol Cell Biochem. 2021 May;476(5):2159-2170. doi: 10.1007/s11010-021-04056-x. Epub 2021 Feb 6. Mol Cell Biochem. 2021. PMID: 33548010 Free PMC article.
-
N-methyl-D-aspartic acid increases tight junction protein destruction in brain endothelial cell via caveolin-1-associated ERK1/2 signaling.Toxicology. 2022 Mar 30;470:153139. doi: 10.1016/j.tox.2022.153139. Epub 2022 Mar 4. Toxicology. 2022. PMID: 35257817
-
Inhibiting Caveolin-1-Related Akt/mTOR Signaling Pathway Protects Against N-methyl-D-Aspartate Receptor Activation-Mediated Dysfunction of Blood-Brain Barrier in vitro.Mol Neurobiol. 2024 Jul;61(7):4166-4177. doi: 10.1007/s12035-023-03833-7. Epub 2023 Dec 8. Mol Neurobiol. 2024. PMID: 38066401 Free PMC article.
-
Caveolin-1 regulates the expression of tight junction proteins during hyperoxia-induced pulmonary epithelial barrier breakdown.Respir Res. 2016 May 12;17(1):50. doi: 10.1186/s12931-016-0364-1. Respir Res. 2016. PMID: 27176222 Free PMC article.
-
The potential mechanisms of Aβ-receptor for advanced glycation end-products interaction disrupting tight junctions of the blood-brain barrier in Alzheimer's disease.Int J Neurosci. 2014 Feb;124(2):75-81. doi: 10.3109/00207454.2013.825258. Epub 2013 Aug 15. Int J Neurosci. 2014. PMID: 23855502 Review.
Cited by
-
HIV-1 Tat Upregulates the Receptor for Advanced Glycation End Products and Superoxide Dismutase-2 in the Heart of Transgenic Mice.Viruses. 2022 Oct 4;14(10):2191. doi: 10.3390/v14102191. Viruses. 2022. PMID: 36298745 Free PMC article.
-
Occludin, caveolin-1, and Alix form a multi-protein complex and regulate HIV-1 infection of brain pericytes.FASEB J. 2020 Dec;34(12):16319-16332. doi: 10.1096/fj.202001562R. Epub 2020 Oct 14. FASEB J. 2020. PMID: 33058236 Free PMC article.
-
Long non‑coding RNA CASC2 suppresses pancreatic cancer cell growth and progression by regulating the miR‑24/MUC6 axis.Int J Oncol. 2020 Feb;56(2):494-507. doi: 10.3892/ijo.2019.4937. Epub 2019 Dec 10. Int J Oncol. 2020. PMID: 31894271 Free PMC article.
-
Systemic exosomal miR-193b-3p delivery attenuates neuroinflammation in early brain injury after subarachnoid hemorrhage in mice.J Neuroinflammation. 2020 Feb 25;17(1):74. doi: 10.1186/s12974-020-01745-0. J Neuroinflammation. 2020. PMID: 32098619 Free PMC article.
-
Disruption of blood-brain barrier: effects of HIV Tat on brain microvascular endothelial cells and tight junction proteins.J Neurovirol. 2023 Dec;29(6):658-668. doi: 10.1007/s13365-023-01179-3. Epub 2023 Oct 29. J Neurovirol. 2023. PMID: 37899420 Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

