P2Y11R regulates cytotoxicity of HBV X protein (HBx) in human normal hepatocytes
- PMID: 31217852
- PMCID: PMC6556625
P2Y11R regulates cytotoxicity of HBV X protein (HBx) in human normal hepatocytes
Abstract
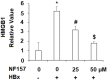
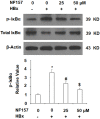
Hepatitis B infection is a major global health problem and a primary cause of hepatocellular carcinoma (HCC). While various antiviral treatments have been explored, there is not yet a reliable method for preventing the progression of chronic hepatitis B infection into HCC. Hepatitis B virus X protein (HBx) plays a major role in viral replication, chronic inflammation and the pathogenicity of chronic liver disease. Modulation of purinergic receptors using their specific agonists has become a popular new strategy for modifying disease processes. In the present study, we investigated the involvement of the P2Y11 receptor using its specific antagonist NF157 in some key aspects of HBx-induced liver disease in human MIHA hepatocytes, including mitochondrial dysfunction due to compromised mitochondrial membrane potential (MMP), oxidative stress resulting from overproduction of reactive oxygen species (ROS) and decreased antioxidant glutathione (GSH), production of proinflammatory cytokines and chemokines such as interleukin (IL)-6, monocyte chemoattractant protein (MCP)-1, and chemokine (C-X-C motif) ligand 2 (CXCL2), as well as activation of cellular signaling pathways including the p38/mitogen-activated protein kinase (p38/MAPK) and nuclear factor-κB (NF-κB) pathways. Our findings present a novel new strategy for the treatment and prevention of chronic liver infection and subsequent morbidities induced by HBx via specific antagonism of the P2Y11 purinergic receptor.
Keywords: HBx; Hepatitis B virus (HBV); NF157; P2Y11; hepatocellular carcinoma; liver disease; purinergic receptors.
Conflict of interest statement
None.
Figures
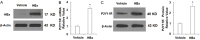





Similar articles
-
Orexin A ameliorates HBV X protein-induced cytotoxicity and inflammatory response in human hepatocytes.Artif Cells Nanomed Biotechnol. 2019 Dec;47(1):2003-2009. doi: 10.1080/21691401.2019.1614014. Artif Cells Nanomed Biotechnol. 2019. PMID: 31106596
-
GPR43 regulates HBV X protein (HBx)-induced inflammatory response in human LO2 hepatocytes.Biomed Pharmacother. 2020 Mar;123:109737. doi: 10.1016/j.biopha.2019.109737. Epub 2019 Dec 26. Biomed Pharmacother. 2020. PMID: 31884344
-
The antagonist of P2Y11 receptor NF157 ameliorates oxidized LDL-induced vascular endothelial inflammation.Artif Cells Nanomed Biotechnol. 2019 Dec;47(1):1839-1845. doi: 10.1080/21691401.2019.1610412. Artif Cells Nanomed Biotechnol. 2019. PMID: 31066305
-
Hepatitis B x (HBx) as a Component of a Functional Cure for Chronic Hepatitis B.Biomedicines. 2022 Sep 7;10(9):2210. doi: 10.3390/biomedicines10092210. Biomedicines. 2022. PMID: 36140311 Free PMC article. Review.
-
Relevance of reactive oxygen species in liver disease observed in transgenic mice expressing the hepatitis B virus X protein.Lab Anim Res. 2020 Feb 26;36:6. doi: 10.1186/s42826-020-00037-1. eCollection 2020. Lab Anim Res. 2020. PMID: 32206612 Free PMC article. Review.
Cited by
-
Purinergic signalling in liver diseases: Pathological functions and therapeutic opportunities.JHEP Rep. 2020 Jul 30;2(6):100165. doi: 10.1016/j.jhepr.2020.100165. eCollection 2020 Dec. JHEP Rep. 2020. PMID: 33103092 Free PMC article. Review.
-
Nonsynonymous C1653T Mutation of Hepatitis B Virus X Gene Enhances Malignancy of Hepatocellular Carcinoma Cells.J Hepatocell Carcinoma. 2022 May 3;9:367-377. doi: 10.2147/JHC.S348690. eCollection 2022. J Hepatocell Carcinoma. 2022. PMID: 35535232 Free PMC article.
-
Cutaneous melanoma and purinergic modulation by phenolic compounds.Purinergic Signal. 2024 Dec;20(6):581-593. doi: 10.1007/s11302-024-10002-5. Epub 2024 Mar 18. Purinergic Signal. 2024. PMID: 38498100 Free PMC article. Review.
References
-
- Schaefer S, Glebe D, Wend UC, Oyunbileg J, Gerlich WH. Universal primers for real-time amplification of DNA from all known Orthohepadnavirus species. J Clin Virol. 2003;27:30–37. - PubMed
-
- Kim HJ, Kim SY, Kim J, Lee H, Choi M, Kim JK, Ahn JK. Hepatitis B virus X protein induces apoptosis by enhancing translocation of Bax to mitochondria. IUBMB Life. 2008;60:473–480. - PubMed
-
- Nair J, Srivatanakul P, Haas C, Jedpiyawongse A, Khuhaprema T, Seitz HK, Bartsch H. High urinary excretion of lipid peroxidation-derived DNA damage in patients with cancer-prone liver diseases. Mutat Res. 2010;683:23–28. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
