Gentamicin-loaded silk/nanosilver composite scaffolds for MRSA-induced chronic osteomyelitis
- PMID: 31218036
- PMCID: PMC6549986
- DOI: 10.1098/rsos.182102
Gentamicin-loaded silk/nanosilver composite scaffolds for MRSA-induced chronic osteomyelitis
Abstract
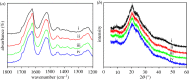
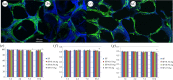
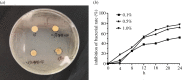
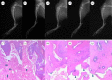
Methicillin-resistant Staphylococcus aureus (MRSA) often induces chronic osteomyelitis and then bone defects. Here, gentamicin-loaded silk/nanosilver composite scaffolds were developed to treat MRSA-induced chronic osteomyelitis. AgNO3 was reduced with silk as a reducing agent in formic acid, forming silver nanoparticles in situ that were distributed uniformly in the composite scaffolds. Superior antibacterial properties against MRSA were achieved for the composite scaffolds, without the compromise of osteogenesis capacity. Then gentamicin was loaded on the scaffolds for better treatment of osteomyelitis. In vivo results showed effective inhibition of the growth of MRSA bacteria, confirming the promising future in the treatment of chronic osteomyelitis.
Keywords: osteomyelitis; silk scaffold; silver nanoparticles.
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Sustainable Release of Vancomycin from Silk Fibroin Nanoparticles for Treating Severe Bone Infection in Rat Tibia Osteomyelitis Model.ACS Appl Mater Interfaces. 2017 Feb 15;9(6):5128-5138. doi: 10.1021/acsami.6b14912. Epub 2017 Feb 6. ACS Appl Mater Interfaces. 2017. PMID: 28106379
-
Evaluation of Silver Ion-Releasing Scaffolds in a 3D Coculture System of MRSA and Human Adipose-Derived Stem Cells for Their Potential Use in Treatment or Prevention of Osteomyelitis.Tissue Eng Part A. 2016 Nov;22(21-22):1258-1263. doi: 10.1089/ten.TEA.2016.0063. Epub 2016 Oct 25. Tissue Eng Part A. 2016. PMID: 27676280 Free PMC article.
-
Preparation of antibacterial degummed silk fiber/nano-hydroxyapatite/polylactic acid composite scaffold by degummed silk fiber loaded silver nanoparticles.Nanotechnology. 2019 Jul 19;30(29):295101. doi: 10.1088/1361-6528/ab13df. Epub 2019 Mar 27. Nanotechnology. 2019. PMID: 30917342
-
Acute haematogenous community-acquired methicillin-resistant Staphylococcus aureus osteomyelitis in an adult: case report and review of literature.BMC Infect Dis. 2012 Oct 25;12:270. doi: 10.1186/1471-2334-12-270. BMC Infect Dis. 2012. PMID: 23098162 Free PMC article. Review.
-
Biodegradable Scaffolds for Bone Regeneration Combined with Drug-Delivery Systems in Osteomyelitis Therapy.Pharmaceuticals (Basel). 2017 Dec 12;10(4):96. doi: 10.3390/ph10040096. Pharmaceuticals (Basel). 2017. PMID: 29231857 Free PMC article. Review.
Cited by
-
[Research progress of nanomaterials in osteomyelitis treatment].Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2021 May 15;35(5):648-655. doi: 10.7507/1002-1892.202012044. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2021. PMID: 33998221 Free PMC article. Chinese.
-
Current approaches for the exploration of antimicrobial activities of nanoparticles.Sci Technol Adv Mater. 2021 Oct 15;22(1):885-907. doi: 10.1080/14686996.2021.1978801. eCollection 2021. Sci Technol Adv Mater. 2021. PMID: 34675754 Free PMC article. Review.
-
Nanobiosystems for Antimicrobial Drug-Resistant Infections.Nanomaterials (Basel). 2021 Apr 22;11(5):1075. doi: 10.3390/nano11051075. Nanomaterials (Basel). 2021. PMID: 33922004 Free PMC article. Review.
-
Antibiotic-Loaded Nano-Sized Delivery Systems: An Insight into Gentamicin and Vancomycin.J Funct Biomater. 2024 Jul 15;15(7):194. doi: 10.3390/jfb15070194. J Funct Biomater. 2024. PMID: 39057315 Free PMC article. Review.
-
Plate-associated localized osteitis in mini-pig by biofilm-forming Methicillin-resistant Staphylococcus aureus (MRSA): establishment of a novel experimental model.Eur J Trauma Emerg Surg. 2022 Aug;48(4):3279-3285. doi: 10.1007/s00068-022-01894-2. Epub 2022 Feb 24. Eur J Trauma Emerg Surg. 2022. PMID: 35201371 Free PMC article.
References
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources

