Homoharringtonine, an approved anti-leukemia drug, suppresses triple negative breast cancer growth through a rapid reduction of anti-apoptotic protein abundance
- PMID: 31218111
- PMCID: PMC6556597
Homoharringtonine, an approved anti-leukemia drug, suppresses triple negative breast cancer growth through a rapid reduction of anti-apoptotic protein abundance
Abstract
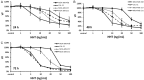
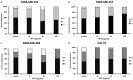
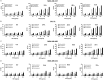
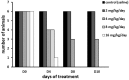
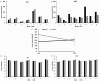
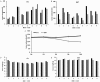
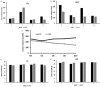
Triple negative breast cancers (TNBC) without BRCA1/2 gene mutation or BRCAness are nowadays the breast malignancies most difficult to treat. Improvement of their treatment, for all phases of the disease, is an important unmet medical need. We analyzed the effect of homoharringtonine (HHT), a natural protein synthesis inhibitor approved for treatment of chronic myeloid leukemia, on four cell lines representing aggressive, BRCA1/2 non-mutated, TNBC genomic categories. We show that HHT inhibits in vitro growth of all cell lines for more than 80%, after 48-72 h exposure to 20-100 ng/mL, the concentrations achievable in human plasma after subcutaneous administration of the drug. HHT, at 100 ng/mL, strongly reduced levels of a major TNBC survival factor, anti-apoptotic protein Mcl-1, after only 2 h of exposure, in all cell lines except MDA-MB-231. Other anti-apoptotic proteins, Bcl-2, survivin and XIAP, were also strongly downregulated. Moreover, in vivo growth of the least sensitive cell line to HHT in vitro, MDA-MB-231, was inhibited for 36.5% in mice, by 1 mg/kg of the drug, given subcutaneously, bi-daily, over 7 days. These results demonstrate marked antineoplastic activity of homoharringtonine in TNBC, making further development of the drug in this disease highly warranted.
Keywords: Homoharringtonine; Mcl-1; breast cancer; protein synthesis; translation inhibitor.
Conflict of interest statement
None.
Figures


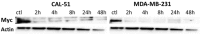
References
-
- Thakur KK, Bordoloi D, Kunnumakkara AB. Alarming burden of triple-negative breast cancer in India. Clin Breast Cancer. 2018;18:e393–e399. - PubMed
-
- Liedtke C, Mazouni C, Hess KR, Andre F, Tordai A, Mejia JA, Symmans WF, Gonzalez-Angulo AM, Hennessy B, Green M, Cristofanilli M, Hortobagyi GN, Pusztai L. Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J. Clin. Oncol. 2008;26:1275–1281. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
