Microglia express GPNMB in the brains of Alzheimer's disease and Nasu-Hakola disease
- PMID: 31218162
- PMCID: PMC6557242
- DOI: 10.5582/irdr.2019.01049
Microglia express GPNMB in the brains of Alzheimer's disease and Nasu-Hakola disease
Abstract
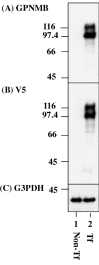
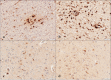
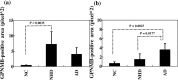
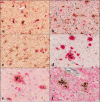
Glycoprotein non-metastatic melanoma protein B (GPNMB) is a type I transmembrane glycoprotein first identified in low-metastatic human melanoma cell lines as a regulator of tumor growth. GPNMB is widely expressed in various tissues, where it is involved in cell differentiation, migration, inflammation/anti-inflammation, tissue regeneration, and neuroprotection. GPNMB is identified in microglia of adult rat brains, neurons and astrocytes of GPNMB transgenic (Tg) mouse brains, and motor neurons of amyotrophic lateral sclerosis (ALS) patients. Nasu-Hakola disease (NHD) is a rare autosomal recessive disorder, characterized by progressive presenile dementia and formation of multifocal bone cysts, caused by genetic mutations of either TYROBP (DAP12) or TREM2. TREM2 and DAP12 constitute a receptor/adaptor signaling complex expressed exclusively on osteoclasts, dendritic cells, macrophages, and microglia. Pathologically, the brains of NHD patients exhibit leukoencephalopathy, astrogliosis, accumulation of axonal spheroids, and remarkable activation of microglia predominantly in the white matter of frontal and temporal lobes and the basal ganglia. At present, molecular mechanisms responsible for development of leukoencephaolpathy in NHD brains remain totally unknown. Recent evidence indicates that disease-associated microglia (DAM) that cluster around amyloid plaques express high levels of GPNMB in Alzheimer's disease (AD) brains. Because microglia act as a key regulator of leukoencephalopathy in NHD brains, it is proposed that GPNMB expressed on microglia might play a protective role in progression of leukoencephalopathy possibly via active phagocytosis of myelin debris. In the present study using immunohistochemistry, we have attempted to clarify the expression of GPNMB in NHD brains, compared with AD brains. We found that microglia accumulating in the white matter express an intense GPNMB immunoreactivity in both NHD and AD brains, suggesting that the accumulation of GPNMB-immunoreactive microglia is a general phenomenon in neurodegenerative brains.
Keywords: Alzheimer's disease; GPNMB; Nasu-Hakola disease; leukoencephalopathy; microglia; osteoactivin.
Figures

Similar articles
-
Alzheimer's disease pathology in Nasu-Hakola disease brains.Intractable Rare Dis Res. 2018 Feb;7(1):32-36. doi: 10.5582/irdr.2017.01088. Intractable Rare Dis Res. 2018. PMID: 29552443 Free PMC article.
-
Microglia express ABI3 in the brains of Alzheimer's disease and Nasu-Hakola disease.Intractable Rare Dis Res. 2017 Nov;6(4):262-268. doi: 10.5582/irdr.2017.01073. Intractable Rare Dis Res. 2017. PMID: 29259854 Free PMC article.
-
Immunohistochemical characterization of microglia in Nasu-Hakola disease brains.Neuropathology. 2011 Aug;31(4):363-75. doi: 10.1111/j.1440-1789.2010.01174.x. Epub 2010 Dec 1. Neuropathology. 2011. PMID: 21118401
-
A novel mutation in TREM2 gene causing Nasu-Hakola disease and review of the literature.Neurobiol Aging. 2017 May;53:194.e13-194.e22. doi: 10.1016/j.neurobiolaging.2017.01.015. Epub 2017 Jan 20. Neurobiol Aging. 2017. PMID: 28214109 Review.
-
The Primary Microglial Leukodystrophies: A Review.Int J Mol Sci. 2022 Jun 6;23(11):6341. doi: 10.3390/ijms23116341. Int J Mol Sci. 2022. PMID: 35683020 Free PMC article. Review.
Cited by
-
Genetic insights into immune mechanisms of Alzheimer's and Parkinson's disease.Front Immunol. 2023 Jun 8;14:1168539. doi: 10.3389/fimmu.2023.1168539. eCollection 2023. Front Immunol. 2023. PMID: 37359515 Free PMC article. Review.
-
Disease and brain region specific immune response profiles in neurodegenerative diseases with pure and mixed protein pathologies.Acta Neuropathol Commun. 2024 Apr 5;12(1):54. doi: 10.1186/s40478-024-01770-7. Acta Neuropathol Commun. 2024. PMID: 38581050 Free PMC article.
-
Potential Utility of Cerebrospinal Fluid Glycoprotein Nonmetastatic Melanoma Protein B as a Neuroinflammatory Diagnostic Biomarker in Mild Cognitive Impairment and Alzheimer's Disease.J Clin Med. 2023 Jul 14;12(14):4689. doi: 10.3390/jcm12144689. J Clin Med. 2023. PMID: 37510803 Free PMC article.
-
The glycoprotein GPNMB protects against oxidative stress through enhanced PI3K/AKT signaling in epidermal keratinocytes.J Biol Chem. 2025 Mar;301(3):108299. doi: 10.1016/j.jbc.2025.108299. Epub 2025 Feb 11. J Biol Chem. 2025. PMID: 39947468 Free PMC article.
-
Emerging targets of α-synuclein spreading in α-synucleinopathies: a review of mechanistic pathways and interventions.Mol Neurodegener. 2025 Jan 23;20(1):10. doi: 10.1186/s13024-025-00797-1. Mol Neurodegener. 2025. PMID: 39849529 Free PMC article. Review.
References
-
- Weterman MA, Ajubi N, van Dinter IM, Degen WG, van Muijen GN, Ruitter DJ, Bloemers HP. nmb, a novel gene, is expressed in low-metastatic human melanoma cell lines and xenografts. Int J Cancer. 1995; 60:73-81. - PubMed
-
- Budge KM, Neal ML, Richardson JR, Safadi FF. Glycoprotein NMB: An emerging r o l e i n neurodegenerative disease. Mol Neurobiol. 2018; 55:5167-5176. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

