Clinical development of triple-combination CFTR modulators for cystic fibrosis patients with one or two F508del alleles
- PMID: 31218221
- PMCID: PMC6571452
- DOI: 10.1183/23120541.00082-2019
Clinical development of triple-combination CFTR modulators for cystic fibrosis patients with one or two F508del alleles
Abstract
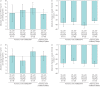
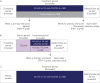
Cystic fibrosis (CF) is caused by mutations in the CF transmembrane conductance regulator gene (CFTR) that result in diminished quantity and/or function of the CFTR anion channel. F508del-CFTR, the most common CF-causing mutation (found in ∼90% of patients), causes severe processing and trafficking defects, resulting in decreased CFTR quantity and function. CFTR modulators are medications that increase the amount of mature CFTR protein (correctors) or enhance channel function (potentiators) at the cell surface. Combinations of CFTR correctors and potentiators (i.e. lumacaftor/ivacaftor, tezacaftor/ivacaftor) have demonstrated clinical benefit in subsets of patients. However, none are approved for patients with CF heterozygous for F508del-CFTR and a minimal function mutation, i.e. a mutation that produces either no protein or protein that is unresponsive to currently approved CFTR modulators. Next-generation CFTR correctors VX-659 and VX-445, each in triple combination with tezacaftor and ivacaftor, improve CFTR processing, trafficking and function in vitro and have demonstrated clinical improvements in phase 2 studies in patients with CF with one or two F508del-CFTR alleles. Here, we present the rationale and design of four randomised phase 3 studies, and their open-label extensions, evaluating VX-659 (ECLIPSE) or VX-445 (AURORA) plus tezacaftor and ivacaftor in patients with one or two F508del-CFTR alleles.
Conflict of interest statement
Conflict of interest: J.L. Taylor-Cousar reports grants and personal fees from Vertex, during the conduct of the study; grants and personal fees from Vertex, Gilead, Celtaxsys and Proteostasis, grants from N30 and Bayer, personal fees from Novartis, Genentech, Protalix and Santhera, outside the submitted work. Conflict of interest: M.A. Mall reports personal fees and nonfinancial support from Vertex, during the conduct of the study; grants from the German Federal Ministry of Education and Research (BMBF) and the Einstein Foundation Berlin, and personal fees from Arrowhead Pharmaceuticals, Bayer, Boehringer Ingelheim, Polyphor, ProQR Therapeutics, PTC Therapeutics, Spyryx Biosciences and Vertex, outside the submitted work. Conflict of interest: B.W. Ramsey reports grants from Vertex, during the conduct of the study. Conflict of interest: E.F. McKone reports grants and personal fees from Vertex, during the conduct of the study; personal fees from Proteostasis, grants from Gilead, and nonfinancial support from Novartis, outside the submitted work. Conflict of interest: E. Tullis reports grants, personal fees and nonfinancial support from Vertex, during the conduct of the study; grants, personal fees and nonfinancial support from Proteostasis and Spyryx, and grants from Corbus and Celtaxys, outside the submitted work. Conflict of interest: G. Marigowda is an employee of Vertex and may hold stock and/or stock options in Vertex; he reports other funding from Vertex during the conduct of the study. Conflict of interest: C.M. McKee is an employee and stockholder of Vertex; she reports other funding from Vertex during the conduct of the study. Conflict of interest: D. Waltz is an employee of Vertex and may hold stock and/or stock options in Vertex; he reports other funding from Vertex during the conduct of the study. He is listed as an inventor on three pending patents: Methods of Treatment of Cystic Fibrosis, Methods of Treatment of Cystic Fibrosis, and Pharmaceutical Compositions for Treating Cystic Fibrosis. Conflict of interest: S.M. Moskowitz is an employee of Vertex and may hold stock and/or stock options in Vertex. He has three patents pending: Methods of Treatment for Cystic Fibrosis, Methods of Treatment of Cystic Fibrosis and Pharmaceutical Compositions for Treating Cystic Fibrosis. Conflict of interest: J. Savage is an employee of Vertex and may hold stock and/or stock options in Vertex; she reports other funding from Vertex during the conduct of the study. Conflict of interest: F. Xuan is an employee of Vertex and may hold stock and/or stock options in Vertex; she reports other funding from Vertex during the conduct of the study. Conflict of interest: S.M. Rowe reports grants and personal fees from Vertex, during the conduct of the study; and grants and personal fees from Bayer, Novartis and Celtaxsys; grants from Forest Research Institute, AstraZeneca, N30/Nivalis, Galapagos/AbbVie, Proteostasis, Eloxx and PTC; and personal fees from Renovion, outside the submitted work.
Figures

References
-
- Cystic Fibrosis Foundation, Johns Hopkins University, The Hospital for Sick Children. Clinical and Functional Translation of CFTR (CFTR2) 2011. www.cftr2.org Date last accessed: January 24, 2019.
-
- Riordan JR, Rommens JM, Kerem B, et al. . Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science 1989; 245: 1066–1073. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
