Catalytic synthesis of 9-cis-retinoids: mechanistic insights
- PMID: 31218312
- PMCID: PMC7004310
- DOI: 10.1039/c9dt02189b
Catalytic synthesis of 9-cis-retinoids: mechanistic insights
Abstract
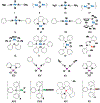
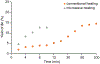
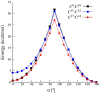
The regioselective Z-isomerization of thermodynamically stable all-trans retinoids remains challenging, and ultimately limits the availability of much needed therapeutics for the treatment of human diseases. We present here a novel, straightforward approach for the catalytic Z-isomerization of retinoids using conventional heat treatment or microwave irradiation. A screen of 20 transition metal-based catalysts identified an optimal approach for the regioselective production of Z-retinoids. The most effective catalytic system was comprised of a palladium complex with labile ligands. Several mechanistic studies, including isotopic H/D exchange and state-of-the-art quantum chemical calculations using coupled cluster methods indicate that the isomerization is initiated by catalyst dimerization followed by the formation of a cyclic, six-membered chloropalladate catalyst-substrate adduct, which eventually opens to produce the desired Z-isomer. The synthetic development described here, combined with thorough mechanistic analysis of the underlying chemistry, highlights the use of readily available transition metal-based catalysts in straightforward formats for gram-scale drug synthesis.
Conflict of interest statement
Conflicts of interest
The authors declare no competing financial interests.
Figures
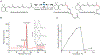





Similar articles
-
Z-isomerization of retinoids through combination of monochromatic photoisomerization and metal catalysis.Org Biomol Chem. 2019 Sep 21;17(35):8125-8139. doi: 10.1039/c9ob01645g. Epub 2019 Aug 28. Org Biomol Chem. 2019. PMID: 31455964 Free PMC article.
-
Nonenzymatic isomerization of all-trans- and 13-cis-retinoids catalyzed by sulfhydryl groups.Drug Metab Dispos. 1986 Nov-Dec;14(6):698-702. Drug Metab Dispos. 1986. PMID: 2877829
-
Isomerization of 11-cis-retinoids to all-trans-retinoids in vitro and in vivo.J Biol Chem. 2001 Dec 21;276(51):48483-93. doi: 10.1074/jbc.M105840200. Epub 2001 Oct 16. J Biol Chem. 2001. PMID: 11604395 Free PMC article.
-
Application of E/Z-Isomerization Technology for Enhancing Processing Efficiency, Health-Promoting Effects, and Usability of Carotenoids: A Review and Future Perspectives.J Oleo Sci. 2022 Feb 3;71(2):151-165. doi: 10.5650/jos.ess21338. Epub 2022 Jan 14. J Oleo Sci. 2022. PMID: 35034944 Review.
-
Computational perspective and evaluation of plausible catalytic mechanisms of peptidyl-prolyl cis-trans isomerases.Biochim Biophys Acta. 2015 Oct;1850(10):1994-2004. doi: 10.1016/j.bbagen.2014.12.023. Epub 2015 Jan 10. Biochim Biophys Acta. 2015. PMID: 25585011 Review.
Cited by
-
Losing, preserving, and restoring vision from neurodegeneration in the eye.Curr Biol. 2023 Oct 9;33(19):R1019-R1036. doi: 10.1016/j.cub.2023.08.044. Curr Biol. 2023. PMID: 37816323 Free PMC article. Review.
-
Pathways and disease-causing alterations in visual chromophore production for vertebrate vision.J Biol Chem. 2021 Jan-Jun;296:100072. doi: 10.1074/jbc.REV120.014405. Epub 2020 Nov 23. J Biol Chem. 2021. PMID: 33187985 Free PMC article. Review.
-
Z-isomerization of retinoids through combination of monochromatic photoisomerization and metal catalysis.Org Biomol Chem. 2019 Sep 21;17(35):8125-8139. doi: 10.1039/c9ob01645g. Epub 2019 Aug 28. Org Biomol Chem. 2019. PMID: 31455964 Free PMC article.
-
Structural basis for the allosteric modulation of rhodopsin by nanobody binding to its extracellular domain.Nat Commun. 2023 Aug 25;14(1):5209. doi: 10.1038/s41467-023-40911-9. Nat Commun. 2023. PMID: 37626045 Free PMC article.
-
Theoretical Study of the Photoisomerization Mechanism of All-Trans-Retinyl Acetate.J Phys Chem A. 2021 Sep 30;125(38):8358-8372. doi: 10.1021/acs.jpca.1c05533. Epub 2021 Sep 21. J Phys Chem A. 2021. PMID: 34546761 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

