Catalytic synthesis of 9-cis-retinoids: mechanistic insights
- PMID: 31218312
- PMCID: PMC7004310
- DOI: 10.1039/c9dt02189b
Catalytic synthesis of 9-cis-retinoids: mechanistic insights
Abstract
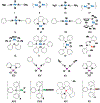
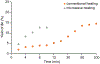
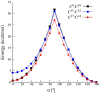
The regioselective Z-isomerization of thermodynamically stable all-trans retinoids remains challenging, and ultimately limits the availability of much needed therapeutics for the treatment of human diseases. We present here a novel, straightforward approach for the catalytic Z-isomerization of retinoids using conventional heat treatment or microwave irradiation. A screen of 20 transition metal-based catalysts identified an optimal approach for the regioselective production of Z-retinoids. The most effective catalytic system was comprised of a palladium complex with labile ligands. Several mechanistic studies, including isotopic H/D exchange and state-of-the-art quantum chemical calculations using coupled cluster methods indicate that the isomerization is initiated by catalyst dimerization followed by the formation of a cyclic, six-membered chloropalladate catalyst-substrate adduct, which eventually opens to produce the desired Z-isomer. The synthetic development described here, combined with thorough mechanistic analysis of the underlying chemistry, highlights the use of readily available transition metal-based catalysts in straightforward formats for gram-scale drug synthesis.
Conflict of interest statement
Conflicts of interest
The authors declare no competing financial interests.
Figures
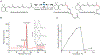





References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

