Mechano-Immunomodulation: Mechanoresponsive Changes in Macrophage Activity and Polarization
- PMID: 31218484
- PMCID: PMC7043232
- DOI: 10.1007/s10439-019-02302-4
Mechano-Immunomodulation: Mechanoresponsive Changes in Macrophage Activity and Polarization
Erratum in
-
Correction to: Mechano-Immunomodulation: Mechanoresponsive Changes in Macrophage Activity and Polarization.Ann Biomed Eng. 2019 Nov;47(11):2341. doi: 10.1007/s10439-019-02326-w. Ann Biomed Eng. 2019. PMID: 31388848
Abstract
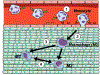
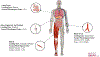
In recent years, biomaterial- and scaffold-based immunomodulation strategies were implemented in tissue regeneration efforts for manipulating macrophage polarization (a.k.a. phenotype or lineage commitment, or differentiation). Yet, most of our understanding of macrophage phenotype commitment and phagocytic capacity is limited to how physical cues (extracellular matrix stiffness, roughness, and topography) and soluble chemical cues (cytokines and chemokines released from the scaffold) influence macrophage polarization. In the context of immune response-tissue interaction, the mechanical cues experienced by the residing cells within the tissue also play a critical role in macrophage polarization and inflammatory response. However, there is no compiled study discussing the effect of the dynamic mechanical environment around the tissues on macrophage polarization and the innate immune response. The aim of this comprehensive review paper is 2-fold; (a) to highlight the importance of mechanical cues on macrophage lineage commitment and function and (b) to summarize the important studies dedicated to understand how macrophage polarization changes with different mechanical loading modalities. For the first time, this review paper compiles and compartmentalizes the studies investigating the role of dynamic mechanical loading with various modalities, amplitude, and frequency on macrophage differentiation. A deeper understanding of macrophage phenotype in mechanically dominant tissues (i.e. musculoskeletal tissues, lung tissues, and cardiovascular tissues) provides mechanistic insights into the design of mechano-immunomodulatory tissue scaffold for tissue regeneration.
Keywords: Anti-inflammatory; Immunomodulation; Macrophages; Mechanical strain; Mechanoimmunomodulation; Mechanotransduction; Phagocytic activity; Polarization; Pro-inflammatory; Tissue engineering.
Figures

References
-
- Spiller KL, Wrona EA, Romero-Torres S, Pallotta I, Graney PL, Witherel CE, Panicker LM, Feldman RA, Urbanska AM, Santambrogio L, Vunjak-Novakovic G, Freytes DO. Differential gene expression in human, murine, and cell line-derived macrophages upon polarization. Exp Cell Res. 2016;347(1):1–13. doi: 10.1016/j.yexcr.2015.10.017. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

