Wnt/β-catenin signaling in brain development and mental disorders: keeping TCF7L2 in mind
- PMID: 31218672
- PMCID: PMC6772062
- DOI: 10.1002/1873-3468.13502
Wnt/β-catenin signaling in brain development and mental disorders: keeping TCF7L2 in mind
Abstract
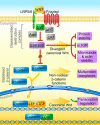
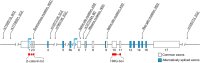
Canonical Wnt signaling, which is transduced by β-catenin and lymphoid enhancer factor 1/T cell-specific transcription factors (LEF1/TCFs), regulates many aspects of metazoan development and tissue renewal. Although much evidence has associated canonical Wnt/β-catenin signaling with mood disorders, the mechanistic links are still unknown. Many components of the canonical Wnt pathway are involved in cellular processes that are unrelated to classical canonical Wnt signaling, thus further blurring the picture. The present review critically evaluates the involvement of classical Wnt/β-catenin signaling in developmental processes that putatively underlie the pathology of mental illnesses. Particular attention is given to the roles of LEF1/TCFs, which have been discussed surprisingly rarely in this context. Highlighting recent discoveries, we propose that alterations in the activity of LEF1/TCFs, and particularly of transcription factor 7-like 2 (TCF7L2), result in defects previously associated with neuropsychiatric disorders, including imbalances in neurogenesis and oligodendrogenesis, the functional disruption of thalamocortical circuitry and dysfunction of the habenula.
Keywords: TCF7L2; Wnt pathway; beta-catenin; brain development; habenula; mental disorders; neurogenesis; oligodendrogenesis; postmitotic differentiation; thalamus.
© 2019 The Authors. FEBS Letters published by John Wiley & Sons Ltd on behalf of Federation of European Biochemical Societies.
Figures


Similar articles
-
Postnatal isoform switch and protein localization of LEF1 and TCF7L2 transcription factors in cortical, thalamic, and mesencephalic regions of the adult mouse brain.Brain Struct Funct. 2013 Nov;218(6):1531-49. doi: 10.1007/s00429-012-0474-6. Epub 2012 Nov 15. Brain Struct Funct. 2013. PMID: 23152144 Free PMC article.
-
Opposing roles of TCF7/LEF1 and TCF7L2 in cyclin D2 and Bmp4 expression and cardiomyocyte cell cycle control during late heart development.Lab Invest. 2019 Jun;99(6):807-818. doi: 10.1038/s41374-019-0204-2. Epub 2019 Feb 18. Lab Invest. 2019. PMID: 30778164 Free PMC article.
-
Transcription Factor 7-like 2 Mediates Canonical Wnt/β-Catenin Signaling and c-Myc Upregulation in Heart Failure.Circ Heart Fail. 2016 Jun;9(6):10.1161/CIRCHEARTFAILURE.116.003010 e003010. doi: 10.1161/CIRCHEARTFAILURE.116.003010. Epub 2016 Jun 14. Circ Heart Fail. 2016. PMID: 27301468 Free PMC article.
-
Canonical Wnt signaling in the oligodendroglial lineage--puzzles remain.Glia. 2015 Oct;63(10):1671-93. doi: 10.1002/glia.22813. Epub 2015 Mar 18. Glia. 2015. PMID: 25782433 Review.
-
Current Understanding on Role of the Wnt Signaling Pathway Effector TCF7L2 in Glucose Homeostasis.Endocr Rev. 2016 Jun;37(3):254-77. doi: 10.1210/er.2015-1146. Epub 2016 May 9. Endocr Rev. 2016. PMID: 27159876 Review.
Cited by
-
Secondary analysis of GenRED data (Genetics of Recurrent Early-Onset major Depression) using MERLIN.Eur Arch Psychiatry Clin Neurosci. 2025 Apr 26. doi: 10.1007/s00406-025-02014-y. Online ahead of print. Eur Arch Psychiatry Clin Neurosci. 2025. PMID: 40285827
-
Impact of MiRNAs on Wnt-related gene activity in breast cancer.Sci Rep. 2025 May 9;15(1):16211. doi: 10.1038/s41598-025-00343-5. Sci Rep. 2025. PMID: 40346182 Free PMC article.
-
Sex-Dependent Shared and Nonshared Genetic Architecture Across Mood and Psychotic Disorders.Biol Psychiatry. 2022 Jan 1;91(1):102-117. doi: 10.1016/j.biopsych.2021.02.972. Epub 2021 Mar 23. Biol Psychiatry. 2022. PMID: 34099189 Free PMC article.
-
TCF7L2 regulates postmitotic differentiation programmes and excitability patterns in the thalamus.Development. 2020 Aug 25;147(16):dev190181. doi: 10.1242/dev.190181. Development. 2020. PMID: 32675279 Free PMC article.
-
Gestational Fisetin Exerts Neuroprotection by Regulating Mitochondria-Directed Canonical Wnt Signaling, BBB Integrity, and Apoptosis in Prenatal VPA-Induced Rodent Model of Autism.Mol Neurobiol. 2024 Jul;61(7):4001-4020. doi: 10.1007/s12035-023-03826-6. Epub 2023 Dec 4. Mol Neurobiol. 2024. PMID: 38048031
References
-
- Yager J and Feinstein RE (2017) Potential applications of the National Institute of Mental Health's Research Domain Criteria (RDoC) to clinical psychiatric practice: how RDoC might be used in assessment, diagnostic processes, case formulation, treatment planning, and clinical notes. J Clin Psychiatry 78, 423–432. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

