Adverse events reported for Mirena levonorgestrel-releasing intrauterine device in France and impact of media coverage
- PMID: 31218710
- PMCID: PMC6710510
- DOI: 10.1111/bcp.14027
Adverse events reported for Mirena levonorgestrel-releasing intrauterine device in France and impact of media coverage
Abstract
Aims: In 2017, concerns regarding adverse events (AEs) associated with the Mirena levonorgestrel intrauterine device were largely echoed in the media in France. This resulted in a tremendous reporting of AEs to pharmacovigilance centres. The aim of this study was to describe the reporting of AEs regarding Mirena in France and to study the impact of media coverage on this reporting.
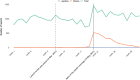
Methods: All cases reports involving Mirena recorded in the French national pharmacovigilance database from marketing (21 July 1995) until 04 August 2017 were extracted. To allow studying the influence of mediatisation, reports were described separately for the periods preceding and following the observed media coverage peak (15 May 2017).
Results: Overall, 3224 reports were considered, 510 (15.8%) recorded before the media coverage peak, and 2714 (84.2%) after. Before the peak, 76.5% of reports originated from health professionals; median time-to-report was of 5.5 months (interquartile range: 1.7-18.6), and median number of AEs per report was 1 (range: 1-17). After the peak, 98.6% originated from patients; median time-to-report was 21 months (interquartile range: 8.1-45.5), and median number of AEs per report was 6 (range: 1-37). After the peak, most reports mentioned anxio-depressive disorders (38.8 vs 10.6% before) or sexual disorders (47.3 vs 6.9%). Other emphasised AEs were weight increase (42.3 vs 10.2%) and pain (gastrointestinal, 19.1 vs 3.5%; musculoskeletal, 22.2 vs 4.5%).
Conclusion: This study highlighted the importance of mediatisation impact on spontaneous reporting with changes concerning amounts of reports, type of reporter, and type of reported AEs. For Mirena, this led to generate signals regarding anxio-depressive and sexual disorders.
Keywords: drug safety; gynaecology/obstetrics; pharmacovigilance.
© 2019 The British Pharmacological Society.
Conflict of interest statement
The study was conducted in the context of a national pharmacovigilance follow‐up for which the Centre de Pharmacovigilance de Bordeaux was appointed by the ANSM. The ANSM had no role in the design and conduct of the study; collection, management, analysis and interpretation of the data; preparation, review or approval of the manuscript; and the decision to submit the manuscript for publication. This publication represents the views of the authors and does not necessarily represent the opinion of the ANSM.
C.L., Am.G., P.B.‐L., Au.G, M.‐C.P.‐P., J.B., G.M., and A.P. have no competing interests to declare.
A.P. is the coordinator of the French DRUGS‐SAFE (DRUGS Systematized Assessment in real‐liFe Environment) national platform of pharmacoepidemiology. This national platform is granted by the ANSM. This work is not part of the DRUGS‐SAFE research programme (
Figures
References
-
- Santé publique France . [Les Françaises et la contraception: premières données du Baromètre santé 2016]. 2017. Available from: https://www.santepubliquefrance.fr/Actualites/Les‐Francaises‐et‐la‐contr.... Accessed December 20, 2018.
-
- [Méthode contraceptives: Focus sur les méthodes les plus efficaces disponibles]. Paris, France: Haute Autorité de Santé. Available from: https://www.has‐sante.fr/portail/upload/docs/application/pdf/2013‐03/syn.... Accessed December 20, 2018.
-
- Winner B, Peipert JF, Zhao Q, et al. Effectiveness of long‐acting reversible contraception. N Engl J Med. 2012;366(21):1998‐2007. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources