The Extracellular Matrix in Ischemic and Nonischemic Heart Failure
- PMID: 31219741
- PMCID: PMC6588179
- DOI: 10.1161/CIRCRESAHA.119.311148
The Extracellular Matrix in Ischemic and Nonischemic Heart Failure
Abstract
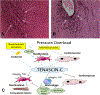
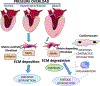
The ECM (extracellular matrix) network plays a crucial role in cardiac homeostasis, not only by providing structural support, but also by facilitating force transmission, and by transducing key signals to cardiomyocytes, vascular cells, and interstitial cells. Changes in the profile and biochemistry of the ECM may be critically implicated in the pathogenesis of both heart failure with reduced ejection fraction and heart failure with preserved ejection fraction. The patterns of molecular and biochemical ECM alterations in failing hearts are dependent on the type of underlying injury. Pressure overload triggers early activation of a matrix-synthetic program in cardiac fibroblasts, inducing myofibroblast conversion, and stimulating synthesis of both structural and matricellular ECM proteins. Expansion of the cardiac ECM may increase myocardial stiffness promoting diastolic dysfunction. Cardiomyocytes, vascular cells and immune cells, activated through mechanosensitive pathways or neurohumoral mediators may play a critical role in fibroblast activation through secretion of cytokines and growth factors. Sustained pressure overload leads to dilative remodeling and systolic dysfunction that may be mediated by changes in the interstitial protease/antiprotease balance. On the other hand, ischemic injury causes dynamic changes in the cardiac ECM that contribute to regulation of inflammation and repair and may mediate adverse cardiac remodeling. In other pathophysiologic conditions, such as volume overload, diabetes mellitus, and obesity, the cell biological effectors mediating ECM remodeling are poorly understood and the molecular links between the primary insult and the changes in the matrix environment are unknown. This review article discusses the role of ECM macromolecules in heart failure, focusing on both structural ECM proteins (such as fibrillar and nonfibrillar collagens), and specialized injury-associated matrix macromolecules (such as fibronectin and matricellular proteins). Understanding the role of the ECM in heart failure may identify therapeutic targets to reduce geometric remodeling, to attenuate cardiomyocyte dysfunction, and even to promote myocardial regeneration.
Keywords: collagen; extracellular matrix; fibroblasts; heart failure; inflammation.
Figures


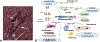
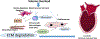
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

