Update: Influenza Activity in the United States During the 2018-19 Season and Composition of the 2019-20 Influenza Vaccine
- PMID: 31220057
- PMCID: PMC6586370
- DOI: 10.15585/mmwr.mm6824a3
Update: Influenza Activity in the United States During the 2018-19 Season and Composition of the 2019-20 Influenza Vaccine
Abstract
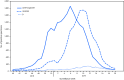
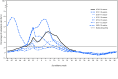
Influenza activity* in the United States during the 2018-19 season (September 30, 2018-May 18, 2019) was of moderate severity (1). Nationally, influenza-like illness (ILI)† activity began increasing in November, peaked during mid-February, and returned to below baseline in mid-April; the season lasted 21 weeks,§ making it the longest season in 10 years. Illness attributed to influenza A viruses predominated, with very little influenza B activity. Two waves of influenza A were notable during this extended season: influenza A(H1N1)pdm09 viruses from October 2018 to mid-February 2019 and influenza A(H3N2) viruses from February through May 2019. Compared with the 2017-18 influenza season, rates of hospitalization this season were lower for adults, but were similar for children. Although influenza activity is currently below surveillance baselines, testing for seasonal influenza viruses and monitoring for novel influenza A virus infections should continue year-round. Receiving a seasonal influenza vaccine each year remains the best way to protect against seasonal influenza and its potentially severe consequences.
Conflict of interest statement
All authors have completed and submitted the ICMJE form for disclosure of potential conflicts of interest. No potential conflicts of interest were disclosed.
Figures
References
-
- World Health Organization. Laboratory methodologies for testing the antiviral susceptibility of influenza viruses: neuraminidase inhibitor (NAI). Geneva, Switzerland: World Health Organization; 2019. https://www.who.int/influenza/gisrs_laboratory/antiviral_susceptibility/...
-
- World Health Organization. Recommended composition of influenza virus vaccines for use in the 2019–2020 northern hemisphere influenza season. Geneva, Switzerland: World Health Organization; 2019. https://www.who.int/influenza/vaccines/virus/recommendations/2019_20_nor...
-
- Food and Drug Administration Center for Biologics Evaluation and Research. Vaccines and Related Biological Products Advisory Committee meeting announcement, March 6–7, 2019. Silver Spring, MD: Department of Health and Human Services, Food and Drug Administration; 2019. https://www.fda.gov/advisory-committees/advisory-committee-calendar/vacc...
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

