Optic nerve regeneration in larval zebrafish exhibits spontaneous capacity for retinotopic but not tectum specific axon targeting
- PMID: 31220164
- PMCID: PMC6586344
- DOI: 10.1371/journal.pone.0218667
Optic nerve regeneration in larval zebrafish exhibits spontaneous capacity for retinotopic but not tectum specific axon targeting
Abstract
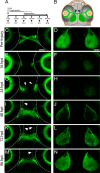
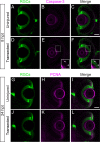
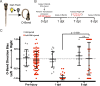
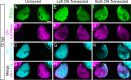
In contrast to mammals, retinal ganglion cells (RGC) axons of the optic nerve even in mature zebrafish exhibit a remarkable capacity for spontaneous regeneration. One constraint of using adult zebrafish is the limited ability to visualize the regeneration process in live animals. To dynamically visualize and trace the degree of target specific optic nerve regeneration, we took advantage of the optical transparency still preserved in post developmental larval zebrafish. We developed a rapid and robust assay to physically transect the larval optic nerve and find that by 96 hours post injury RGC axons have robustly regrown onto the optic tectum. We observe functional regeneration by 8 days post injury, and demonstrate that similar to adult zebrafish, optic nerve transection in larval zebrafish does not prominently induce cell death or proliferation of RGC neurons. Furthermore, we find that partial optic nerve transection results in axonal growth predominantly to the original, contralateral tectum, while complete transection results in innervation of both the correct contralateral and 'incorrect' ipsilateral tectum. Axonal tracing reveals that although regenerating axons innervate the 'incorrect' ipsilateral tectum, they successfully target their topographically appropriate synaptic areas. Combined, our results validate post developmental larval zebrafish as a powerful model for optic nerve regeneration, and reveal intricate mechanistic differences between axonal growth, midline guidance and synaptic targeting during zebrafish optic nerve regeneration.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
Assaying Optic Nerve Regeneration in Larval Zebrafish.Methods Mol Biol. 2023;2636:191-203. doi: 10.1007/978-1-0716-3012-9_10. Methods Mol Biol. 2023. PMID: 36881301
-
Precise lamination of retinal axons generates multiple parallel input pathways in the tectum.J Neurosci. 2013 Mar 13;33(11):5027-39. doi: 10.1523/JNEUROSCI.4990-12.2013. J Neurosci. 2013. PMID: 23486973 Free PMC article.
-
Axonal transport of proteoglycans in regenerating goldfish optic nerve.Exp Neurol. 1994 Mar;126(1):129-37. doi: 10.1006/exnr.1994.1049. Exp Neurol. 1994. PMID: 7512512
-
Exploring Optic Nerve Axon Regeneration.Curr Neuropharmacol. 2017;15(6):861-873. doi: 10.2174/1570159X14666161227150250. Curr Neuropharmacol. 2017. PMID: 28029073 Free PMC article. Review.
-
Trying to understand axonal regeneration in the CNS of fish.J Neurobiol. 1992 Jul;23(5):537-50. doi: 10.1002/neu.480230508. J Neurobiol. 1992. PMID: 1431836 Review.
Cited by
-
Concept of Normativity in Multi-Omics Analysis of Axon Regeneration.Biomolecules. 2024 Jun 21;14(7):735. doi: 10.3390/biom14070735. Biomolecules. 2024. PMID: 39062450 Free PMC article.
-
Serotonin neuromodulation directs optic nerve regeneration.Development. 2025 Jul 1;152(13):dev204334. doi: 10.1242/dev.204334. Epub 2025 Jul 7. Development. 2025. PMID: 40521655 Free PMC article.
-
The transcription factor Jun is necessary for optic nerve regeneration in larval zebrafish.PLoS One. 2025 Mar 10;20(3):e0313534. doi: 10.1371/journal.pone.0313534. eCollection 2025. PLoS One. 2025. PMID: 40063628 Free PMC article.
-
Assaying Optic Nerve Regeneration in Larval Zebrafish.Methods Mol Biol. 2023;2636:191-203. doi: 10.1007/978-1-0716-3012-9_10. Methods Mol Biol. 2023. PMID: 36881301
-
Transcriptional control of visual neural circuit development by GS homeobox 1.PLoS Genet. 2024 Apr 26;20(4):e1011139. doi: 10.1371/journal.pgen.1011139. eCollection 2024 Apr. PLoS Genet. 2024. PMID: 38669217 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

