Neutrophil and macrophage influx into the central nervous system are inflammatory components of lethal Rift Valley fever encephalitis in rats
- PMID: 31220182
- PMCID: PMC6605717
- DOI: 10.1371/journal.ppat.1007833
Neutrophil and macrophage influx into the central nervous system are inflammatory components of lethal Rift Valley fever encephalitis in rats
Abstract
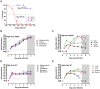
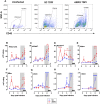
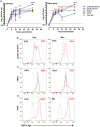
Rift Valley fever virus (RVFV) causes severe disease in livestock concurrent with zoonotic transmission to humans. A subset of people infected with RVFV develop encephalitis, and significant gaps remain in our knowledge of how RVFV causes pathology in the brain. We previously found that, in Lewis rats, subcutaneous inoculation with RVFV resulted in subclinical disease while inhalation of RVFV in a small particle aerosol caused fatal encephalitis. Here, we compared the disease course of RVFV in Lewis rats after each different route of inoculation in order to understand more about pathogenic mechanisms of fatal RVFV encephalitis. In aerosol-infected rats with lethal encephalitis, neutrophils and macrophages were the major cell types infiltrating the CNS, and this was concomitant with microglia activation and extensive cytokine inflammation. Despite this, prevention of neutrophil infiltration into the brain did not ameliorate disease. Unexpectedly, in subcutaneously-inoculated rats with subclinical disease, detectable viral RNA was found in the brain along with T-cell infiltration. This study sheds new light on the pathogenic mechanisms of RVFV encephalitis.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
Rift valley Fever virus encephalitis is associated with an ineffective systemic immune response and activated T cell infiltration into the CNS in an immunocompetent mouse model.PLoS Negl Trop Dis. 2014 Jun 12;8(6):e2874. doi: 10.1371/journal.pntd.0002874. eCollection 2014 Jun. PLoS Negl Trop Dis. 2014. PMID: 24922480 Free PMC article.
-
Vascular permeability in the brain is a late pathogenic event during Rift Valley fever virus encephalitis in rats.Virology. 2019 Jan 2;526:173-179. doi: 10.1016/j.virol.2018.10.021. Epub 2018 Nov 2. Virology. 2019. PMID: 30396029 Free PMC article.
-
Development of Rift valley fever encephalitis in rats is mediated by early infection of olfactory epithelium and neuroinvasion across the cribriform plate.J Gen Virol. 2021 Feb;102(2):001522. doi: 10.1099/jgv.0.001522. J Gen Virol. 2021. PMID: 33231535 Free PMC article.
-
Rift Valley Fever Virus Encephalitis: Viral and Host Determinants of Pathogenesis.Annu Rev Virol. 2024 Sep;11(1):309-325. doi: 10.1146/annurev-virology-093022-011544. Epub 2024 Aug 30. Annu Rev Virol. 2024. PMID: 38635867 Review.
-
Advances in Understanding Neuropathogenesis of Rift Valley Fever Virus.Annu Rev Virol. 2022 Sep 29;9(1):437-450. doi: 10.1146/annurev-virology-091919-065806. Annu Rev Virol. 2022. PMID: 36173701 Free PMC article. Review.
Cited by
-
Spatiotemporal perturbations of the plasminogen activation system in a rat model of acute organophosphate intoxication.Acta Neuropathol Commun. 2025 Mar 18;13(1):62. doi: 10.1186/s40478-025-01979-0. Acta Neuropathol Commun. 2025. PMID: 40102979 Free PMC article.
-
Acute Rift Valley fever virus infection induces inflammatory cytokines and cell death in ex vivo rat brain slice culture.J Gen Virol. 2024 Mar;105(3):001970. doi: 10.1099/jgv.0.001970. J Gen Virol. 2024. PMID: 38546100 Free PMC article.
-
Intranasal Exposure to Rift Valley Fever Virus Live-Attenuated Strains Leads to High Mortality Rate in Immunocompetent Mice.Viruses. 2022 Nov 8;14(11):2470. doi: 10.3390/v14112470. Viruses. 2022. PMID: 36366567 Free PMC article.
-
Gene co-expression network identifies critical genes, pathways and regulatory motifs mediating the progression of rift valley fever in Bostaurus.Heliyon. 2023 Jul 14;9(7):e18175. doi: 10.1016/j.heliyon.2023.e18175. eCollection 2023 Jul. Heliyon. 2023. PMID: 37519716 Free PMC article.
-
The lipopeptide Pam3CSK4 inhibits Rift Valley fever virus infection and protects from encephalitis.PLoS Pathog. 2024 Jun 27;20(6):e1012343. doi: 10.1371/journal.ppat.1012343. eCollection 2024 Jun. PLoS Pathog. 2024. PMID: 38935789 Free PMC article.
References
-
- World Health Organization. Rift Valley fever–Kenya 2018 [6/18/18]. https://www.who.int/csr/don/18-june-2018-rift-valley-fever-kenya/en/.
-
- World Health Organization. Rift Valley fever–Gambia 2018 [cited 2018 3/16/18]. http://www.who.int/csr/don/26-february-2018-rift-valley-fever-gambia/en/.
-
- World Health Organization. South Sudan declares Rift Valley fever outbreak in parts of Eastern Lakes State 2018 [updated March 12, 2018; cited 2018 3/16/18]. http://www.afro.who.int/news/south-sudan-declares-rift-valley-fever-outb....
-
- World Health Organization. Annual review of diseases prioritized under the Research and Development Blueprint. 2017 January 24–25, 2017. Report No.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

