Ledipasvir/Sofosbuvir for 8 Weeks to Treat Acute Hepatitis C Virus Infections in Men With Human Immunodeficiency Virus Infections: Sofosbuvir-Containing Regimens Without Interferon for Treatment of Acute HCV in HIV-1 Infected Individuals
- PMID: 31220220
- PMCID: PMC6637278
- DOI: 10.1093/cid/ciy913
Ledipasvir/Sofosbuvir for 8 Weeks to Treat Acute Hepatitis C Virus Infections in Men With Human Immunodeficiency Virus Infections: Sofosbuvir-Containing Regimens Without Interferon for Treatment of Acute HCV in HIV-1 Infected Individuals
Abstract
Background: Current guidelines for the management of hepatitis C virus (HCV) infections provide varying recommendations for the optimal treatment of acute HCV infections. There are limited data from small cohort studies to provide guidance on the best approach to treatment of this important patient population.
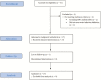
Methods: Sofosbuvir-Containing Regimens Without Interferon for Treatment of Acute HCV in HIV-1 Infected Individuals is an open-label, 2-cohort, Phase 1 clinical trial in which the second cohort assessed the safety and efficacy of 8 weeks of ledipasvir/sofosbuvir for the treatment of acute HCV infections in participants with chronic human immunodeficiency virus (HIV)-1 infections. This final analysis of the second cohort had a planned accrual of 27 participants, based on non-inferiority criteria, compared to the study-defined, historical, sustained virologic response (SVR) of 60% with pegylated-interferon/ribavirin.
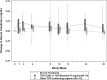
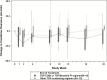
Results: We enrolled 27 men (9 Hispanic; 11 White, non-Hispanic; 5 Black, non-Hispanic; 2 Asian or Pacific Islander; median age 46 years). Most (96%) had HCV genotype-1 infection and 59% had the favorable interleukin 28B CC genotype. The median baseline HCV RNA load was 6.17 log10 IU/mL (interquartile range 4.51 - 6.55). All participants (100%) achieved the primary outcome of a sustained virologic response 12 weeks after the date of the last dose of study treatment (90% confidence interval 90-100%), achieving non-inferiority versus the 60% historic benchmark. No treatment discontinuations occurred.
Conclusions: This multicenter clinical trial, investigating 8 weeks of ledipasvir/sofosbuvir for acute HCV infections in men with HIV infections, reports a 100% SVR. This study provides the rationale for larger studies of shortened courses of direct-acting antiviral therapies in persons with HIV infections, including those with high baseline HCV RNA loads.
Clinical trials registration: NCT02128217.
Keywords: direct-acting antivirals; early infection; human immunodeficiency virus; interferon-free; men who have sex with men.
© The Author(s) 2019. Published by Oxford University Press for the Infectious Diseases Society of America. All rights reserved. For permissions, e-mail: journals.permissions@oup.com.
Figures

References
-
- World Health Organization. Global Hepatitis Report 2017 [License: CC BY-NC-SA 3.0IGO]. Geneva, Switzerland: World Health Organization; 2017.
-
- Zibbell JE , Iqbal K , Patel RC , et al. ; Centers for Disease Control and Prevention Increases in hepatitis C virus infection related to injection drug use among persons aged ≤30 years - Kentucky, Tennessee, Virginia, and West Virginia, 2006-2012. MMWR Morb Mortal Wkly Rep 2015; 64:453–8. - PMC - PubMed
-
- EASL Recommendations on Treatment of Hepatitis C 2016. J Hepatol 2016; h.d.d.o.j. - PubMed
-
- American Association for the Study of Liver Disease, Infectious Diseases Society of America . Recommendations for testing, managing, and treating hepatitis C Available at: http://www.hcvguidelines.org/ - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- UM1 AI069503/AI/NIAID NIH HHS/United States
- U01 AI069471/AI/NIAID NIH HHS/United States
- P30 AI045008/AI/NIAID NIH HHS/United States
- U01 AI068636/AI/NIAID NIH HHS/United States
- UM1 AI069496/AI/NIAID NIH HHS/United States
- UL1 TR002384/TR/NCATS NIH HHS/United States
- UM1 AI069465/AI/NIAID NIH HHS/United States
- UM1 AI069419/AI/NIAID NIH HHS/United States
- U01 AI069419/AI/NIAID NIH HHS/United States
- UL1 TR000457/TR/NCATS NIH HHS/United States
- T32 DK007191/DK/NIDDK NIH HHS/United States
- UM1 AI069412/AI/NIAID NIH HHS/United States
- UM1 AI068634/AI/NIAID NIH HHS/United States
- R01 DA041298/DA/NIDA NIH HHS/United States
- UM1 AI106701/AI/NIAID NIH HHS/United States

