Revisiting gametocyte biology in malaria parasites
- PMID: 31220244
- PMCID: PMC6606849
- DOI: 10.1093/femsre/fuz010
Revisiting gametocyte biology in malaria parasites
Erratum in
-
Corrigendum to: Revisiting gametocyte biology in malaria parasites.FEMS Microbiol Rev. 2019 Nov 1;43(6):684. doi: 10.1093/femsre/fuz020. FEMS Microbiol Rev. 2019. PMID: 31386107 Free PMC article. No abstract available.
Abstract
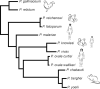
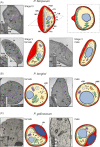
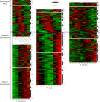
Gametocytes are the only form of the malaria parasite that is transmissible to the mosquito vector. They are present at low levels in blood circulation and significant knowledge gaps exist in their biology. Recent reductions in the global malaria burden have brought the possibility of elimination and eradication, with renewed focus on malaria transmission biology as a basis for interventions. This review discusses recent insights into gametocyte biology in the major human malaria parasite, Plasmodium falciparum and related species.
Keywords: Plasmodium falciparum; gametocyte; malaria; transmission.
© FEMS 2019.
Figures

References
-
- Aikawa M, Huff CG, Sprinz H. Comparative fine structure study of the gametocytes of avian, reptilian, and mammalian malarial parasites. J Ultrastruct Res. 1969;26:316–31. - PubMed
-
- Alano P, Roca L, Smith Det al. .. Plasmodium falciparum: parasites defective in early stages of gametocytogenesis. Exp Parasitol. 1995;81:227–35. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

