Functional Hypogonadotropic Hypogonadism in Men: Underlying Neuroendocrine Mechanisms and Natural History
- PMID: 31220265
- PMCID: PMC6594303
- DOI: 10.1210/jc.2018-02697
Functional Hypogonadotropic Hypogonadism in Men: Underlying Neuroendocrine Mechanisms and Natural History
Abstract
Context: After completion of puberty a subset of men experience functional hypogonadotropic hypogonadism (FHH) secondary to excessive exercise or weight loss. This phenomenon is akin to hypothalamic amenorrhea (HA) in women, yet little is known about FHH in men.
Objective: To investigate the neuroendocrine mechanisms, genetics, and natural history underlying FHH.
Design: Retrospective study in an academic medical center.
Participants: Healthy postpubertal men presenting with symptoms of hypogonadism in the setting of excessive exercise (>10 hours/week) or weight loss (>10% of body weight). Healthy age-matched men served as controls.
Interventions: Clinical assessment, biochemical and neuroendocrine profiling, body composition, semen analysis, and genetic evaluation of genes known to cause isolated GnRH deficiency.
Main outcome measures: Reproductive hormone levels, endogenous GnRH-induced LH pulse patterns, and rare genetic variants.
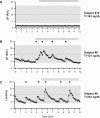
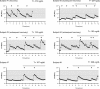
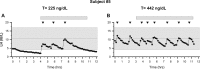
Results: Ten men with FHH were compared with 18 age-matched controls. Patients had significantly lower body mass index, testosterone, LH, and mean LH pulse amplitudes yet normal LH pulse frequency, serum FSH, and sperm counts. Some patients exhibited nocturnal, sleep-entrained LH pulses characteristic of early puberty, and one FHH subject showed a completely apulsatile LH secretion. After decreased exercise and weight gain, five men with men had normalized serum testosterone levels, and symptoms resolved. Rare missense variants in NSMF (n = 1) and CHD7 (n = 1) were identified in two men with FHH.
Conclusions: FHH is a rare, reversible form of male GnRH deficiency. LH pulse patterns in male FHH are similar to those observed in women with HA. This study expands the spectrum of GnRH deficiency disorders in men.
Copyright © 2019 Endocrine Society.
Figures
Similar articles
-
Exaggerated free alpha-subunit levels during pulsatile gonadotropin-releasing hormone replacement in women with idiopathic hypogonadotropic hypogonadism.J Clin Endocrinol Metab. 1998 Jan;83(1):241-7. doi: 10.1210/jcem.83.1.4488. J Clin Endocrinol Metab. 1998. PMID: 9435449 Clinical Trial.
-
The spectrum of abnormal patterns of gonadotropin-releasing hormone secretion in men with idiopathic hypogonadotropic hypogonadism: clinical and laboratory correlations.J Clin Endocrinol Metab. 1987 Feb;64(2):283-91. doi: 10.1210/jcem-64-2-283. J Clin Endocrinol Metab. 1987. PMID: 3098771
-
Gonadotropin-releasing hormone (GnRH) physiology in men and women.Acta Med Hung. 1986;43(2):201-21. Acta Med Hung. 1986. PMID: 3295743
-
Hormonal control of spermatogenesis in men: therapeutic aspects in hypogonadotropic hypogonadism.Ann Endocrinol (Paris). 2014 May;75(2):98-100. doi: 10.1016/j.ando.2014.04.002. Epub 2014 Apr 29. Ann Endocrinol (Paris). 2014. PMID: 24793994 Review.
-
Mutations of the GnRH receptor gene: a new cause of autosomal-recessive hypogonadotropic hypogonadism.Arch Med Res. 1999 Nov-Dec;30(6):481-5. doi: 10.1016/s0188-4409(99)00072-7. Arch Med Res. 1999. PMID: 10714361 Review.
Cited by
-
Variety of genetic defects in GnRH and hypothalamic-pituitary signaling and development in normosmic patients with IHH.Front Endocrinol (Lausanne). 2024 Jul 1;15:1396805. doi: 10.3389/fendo.2024.1396805. eCollection 2024. Front Endocrinol (Lausanne). 2024. PMID: 39010903 Free PMC article.
-
Expanding the Spectrum of Endocrine Abnormalities Associated With SOX11-related Disorders.J Clin Endocrinol Metab. 2025 Mar 17;110(4):1044-1052. doi: 10.1210/clinem/dgae620. J Clin Endocrinol Metab. 2025. PMID: 39290158
-
Relative energy deficiency in sports (RED-S): elucidation of endocrine changes affecting the health of males and females.Hormones (Athens). 2021 Mar;20(1):35-47. doi: 10.1007/s42000-020-00214-w. Epub 2020 Jun 17. Hormones (Athens). 2021. PMID: 32557402 Review.
-
Gene-environment interaction in functional hypothalamic amenorrhea.Front Endocrinol (Lausanne). 2024 Aug 29;15:1423898. doi: 10.3389/fendo.2024.1423898. eCollection 2024. Front Endocrinol (Lausanne). 2024. PMID: 39268244 Free PMC article. Review.
-
Recent Update on the Molecular Mechanisms of Gonadal Steroids Action in Adipose Tissue.Int J Mol Sci. 2021 May 14;22(10):5226. doi: 10.3390/ijms22105226. Int J Mol Sci. 2021. PMID: 34069293 Free PMC article. Review.
References
-
- Santoro N, Filicori M, Crowley WF Jr. Hypogonadotropic disorders in men and women: diagnosis and therapy with pulsatile gonadotropin-releasing hormone. Endocr Rev. 1986;7(1):11–23. - PubMed
-
- Perkins RB, Hall JE, Martin KA. Neuroendocrine abnormalities in hypothalamic amenorrhea: spectrum, stability, and response to neurotransmitter modulation. J Clin Endocrinol Metab. 1999;84(6):1905–1911. - PubMed
-
- Otis CL, Drinkwater B, Johnson M, Loucks A, Wilmore J. American College of Sports Medicine position stand. The female athlete triad. Med Sci Sports Exerc. 1997;29(5):i–ix. - PubMed
-
- Caronia LM, Martin C, Welt CK, Sykiotis GP, Quinton R, Thambundit A, Avbelj M, Dhruvakumar S, Plummer L, Hughes VA, Seminara SB, Boepple PA, Sidis Y, Crowley WF Jr, Martin KA, Hall JE, Pitteloud N. A genetic basis for functional hypothalamic amenorrhea. N Engl J Med. 2011;364(3):215–225. - PMC - PubMed

