Basal cell carcinoma characterization using fusion ex vivo confocal microscopy: a promising change in conventional skin histopathology
- PMID: 31220341
- PMCID: PMC6923630
- DOI: 10.1111/bjd.18239
Basal cell carcinoma characterization using fusion ex vivo confocal microscopy: a promising change in conventional skin histopathology
Abstract
Background: Ex vivo confocal microscopy (CM) works under two modes, fluorescence and reflectance, allowing the visualization of different structures. Fluorescence CM (FCM) requires a contrast agent and has been used for the analysis of basal cell carcinomas (BCCs) during Mohs surgery. Conversely, reflectance CM (RCM) is mostly used for in vivo diagnosis of equivocal skin tumours. Recently, a new, faster ex vivo confocal microscope has been developed which simultaneously uses both lasers (fusion mode).
Objectives: To describe the BCC features identified on reflectance, fluorescence and fusion modes using this novel device. To determine the best mode to identify characteristic BCC features. To develop a new staining protocol to improve the visualization of BCC under the different modes.
Methods: From September 2016 to June 2017, we prospectively included consecutive BCCs which were excised using Mohs surgery in our department. The lesions were evaluated using ex vivo CM after routine Mohs surgery. The specimens were first stained with acridine orange and then stained using both acetic acid and acridine orange.
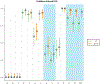
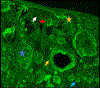
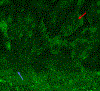
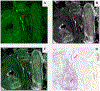
Results: We included 78 BCCs (35 infiltrative, 25 nodular, 12 micronodular, 6 superficial). Most features were better visualized with the fusion mode using the double staining. We also identified new CM ex vivo features, dendritic and plump cells, which have not been reported previously.
Conclusions: Our results suggest that nuclei characteristics are better visualized in FCM but cytoplasm and surrounding stroma are better visualized in RCM. Thus, the simultaneous evaluation of reflectance and fluorescence seems to be beneficial due to its complementary effect. What's already known about this topic? Ex vivo fluorescent confocal microscopy (FCM) is an imaging technique that allows histopathological analysis of fresh tissue. FCM is faster - at least one-third of the time - than conventional methods. FCM has a sensitivity of 88% and a specificity of 99% in detecting basal cell carcinomas (BCCs). What does this study add? Reflectance and fluorescence modes can be used simultaneously in a new ex vivo CM device. Each mode complements the other, resulting in an increase in the detection of BCC features in fusion mode. A combined staining using acetic acid and acridine orange enhances the visualization of tumour and stroma without damaging the tissue for further histopathological analysis.
© 2019 British Association of Dermatologists.
Conflict of interest statement
Figures













Comment in
-
The road to real-time, bedside, optical imaging pathology: basal cell carcinoma and beyond.Br J Dermatol. 2020 Feb;182(2):257-259. doi: 10.1111/bjd.18471. Br J Dermatol. 2020. PMID: 32017020 No abstract available.
Similar articles
-
Detection of basal cell carcinomas in Mohs excisions with fluorescence confocal mosaicing microscopy.Br J Dermatol. 2009 Jun;160(6):1242-50. doi: 10.1111/j.1365-2133.2009.09141.x. Epub 2009 Mar 30. Br J Dermatol. 2009. PMID: 19416248 Free PMC article.
-
Evaluating ex vivo fluorescence confocal microscopy images of basal cell carcinomas in Mohs excised tissue.Br J Dermatol. 2014 Sep;171(3):561-70. doi: 10.1111/bjd.13070. Epub 2014 Aug 13. Br J Dermatol. 2014. PMID: 24749970
-
Ex-vivo fluorescence confocal microscopy with digital staining for characterizing basal cell carcinoma on frozen sections: A comparison with histology.J Biophotonics. 2021 Aug;14(8):e202100094. doi: 10.1002/jbio.202100094. Epub 2021 May 24. J Biophotonics. 2021. PMID: 33991061
-
Role of In Vivo Reflectance Confocal Microscopy in the Analysis of Melanocytic Lesions.Acta Dermatovenerol Croat. 2018 Apr;26(1):64-67. Acta Dermatovenerol Croat. 2018. PMID: 29782304 Review.
-
In Vivo and Ex Vivo Confocal Microscopy for Dermatologic and Mohs Surgeons.Dermatol Clin. 2016 Oct;34(4):497-504. doi: 10.1016/j.det.2016.05.012. Dermatol Clin. 2016. PMID: 27692455 Free PMC article. Review.
Cited by
-
A Feasibility Study for Immediate Histological Assessment of Various Skin Biopsies Using Ex Vivo Confocal Laser Scanning Microscopy.Diagnostics (Basel). 2022 Dec 2;12(12):3030. doi: 10.3390/diagnostics12123030. Diagnostics (Basel). 2022. PMID: 36553036 Free PMC article.
-
Role of VivaScope 2500 ex vivo confocal microscopy in skin pathology: Advantages, limitations, and future prospects.Skin Res Technol. 2023 Jun;29(6):e13388. doi: 10.1111/srt.13388. Skin Res Technol. 2023. PMID: 37357649 Free PMC article.
-
One-Stop Shop: Diagnosis and Treatment of Basal Cell Carcinoma in One Step.J Clin Med. 2024 Jun 29;13(13):3830. doi: 10.3390/jcm13133830. J Clin Med. 2024. PMID: 38999395 Free PMC article. Review.
-
[Giant nodule and keratinized facial basal cell carcinoma: a case report].Lin Chuang Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2022 Apr;36(4):307-309. doi: 10.13201/j.issn.2096-7993.2022.04.015. Lin Chuang Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2022. PMID: 35511627 Free PMC article. Chinese.
-
Ex vivo confocal microscopy for surgical margin assessment: A histology-compared study on 109 specimens.Skin Health Dis. 2022 Jan 10;2(2):e91. doi: 10.1002/ski2.91. eCollection 2022 Jun. Skin Health Dis. 2022. PMID: 35677928 Free PMC article.
References
-
- Bennàssar A, Vilata A, Puig S, Malvehy J. Ex vivo fluorescence confocal microscopy for fast evaluation of tumour margins during Mohs surgery. Br J Dermatol 2014; 170:360–5. - PubMed

