The Protective Role of Natriuretic Peptide Receptor 2 against High Salt Injury in the Renal Papilla
- PMID: 31220449
- PMCID: PMC6724224
- DOI: 10.1016/j.ajpath.2019.05.020
The Protective Role of Natriuretic Peptide Receptor 2 against High Salt Injury in the Renal Papilla
Abstract
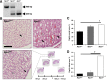
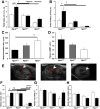
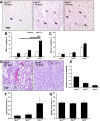
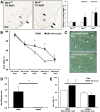
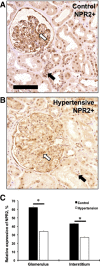
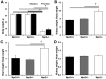
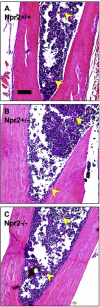
Mutations in natriuretic peptide receptor 2 (Npr2) gene cause a rare form of short-limbed dwarfism, but its physiological effects have not been well studied. Human and mouse genetic data suggest that Npr2 in the kidney plays a role in salt homeostasis. Herein, we described anatomic changes within renal papilla of Npr2 knockout (Npr2-/-) mice. Dramatic reduction was found in diuresis, and albuminuria was evident after administration of 1% NaCl in drinking water in Npr2-/- and heterozygous (Npr2+/-) mice compared with their wild-type (Npr2+/+) littermates. There was indication of renal epithelial damage accompanied by high numbers of red blood cells and inflammatory cells (macrophage surface glycoproteins binding to galectin-3) and an increase of renal epithelial damage marker (T-cell Ig and mucin domain 1) in Npr2-/- mice. Addition of 1% NaCl tended to increase apoptotic cells (cleaved caspase 3) in the renal papilla of Npr2-/- mice. In vitro, genetic silencing of the Npr2 abolished protective effects of C-type natriuretic peptide, a ligand for Npr2, against death of M-1 kidney epithelial cells exposed to 360 mmol/L NaCl. Finally, significantly lower levels of expression of the NPR2 protein were detected in renal samples of hypertensive compared with normotensive human subjects. Taken together, these findings suggest that Npr2 is essential to protect renal epithelial cells from high concentrations of salt and prevent kidney injury.
Copyright © 2019 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures
Similar articles
-
Deficiency of Natriuretic Peptide Receptor 2 Promotes Bicuspid Aortic Valves, Aortic Valve Disease, Left Ventricular Dysfunction, and Ascending Aortic Dilatations in Mice.Circ Res. 2018 Feb 2;122(3):405-416. doi: 10.1161/CIRCRESAHA.117.311194. Epub 2017 Dec 22. Circ Res. 2018. PMID: 29273600
-
Atrial natriuretic peptide receptors in renal papilla of DOCA-salt hypertensive rats.Am J Physiol. 1990 Jul;259(1 Pt 2):F130-7. doi: 10.1152/ajprenal.1990.259.1.F130. Am J Physiol. 1990. PMID: 2165357
-
Heterozygous mutations in natriuretic peptide receptor-B (NPR2) gene as a cause of short stature in patients initially classified as idiopathic short stature.J Clin Endocrinol Metab. 2013 Oct;98(10):E1636-44. doi: 10.1210/jc.2013-2142. Epub 2013 Sep 3. J Clin Endocrinol Metab. 2013. PMID: 24001744
-
The renal paracrine peptide system--possible urologic implications of urodilatin.World J Urol. 1996;14(6):375-9. doi: 10.1007/BF00183118. World J Urol. 1996. PMID: 8986039 Review.
-
Nppc/Npr2/cGMP signaling cascade maintains oocyte developmental capacity.Cell Mol Biol (Noisy-le-grand). 2019 Apr 30;65(4):83-89. Cell Mol Biol (Noisy-le-grand). 2019. PMID: 31078160 Review.
Cited by
-
Natriuretic Peptide Receptor 2 Locus Contributes to Carotid Remodeling.J Am Heart Assoc. 2020 May 18;9(10):e014257. doi: 10.1161/JAHA.119.014257. Epub 2020 May 12. J Am Heart Assoc. 2020. PMID: 32394795 Free PMC article.
-
Localization of natriuretic peptide receptors A, B, and C in healthy and diseased mouse kidneys.Pflugers Arch. 2023 Mar;475(3):343-360. doi: 10.1007/s00424-022-02774-9. Epub 2022 Dec 8. Pflugers Arch. 2023. PMID: 36480070 Free PMC article.
References
-
- Kinnunen P., Vuolteenaho O., Ruskoaho H. Mechanisms of atrial and brain natriuretic peptide release from rat ventricular myocardium: effect of stretching. Endocrinology. 1993;132:1961–1970. - PubMed
-
- Rosenzweig A., Seidman C.E. Atrial natriuretic factor and related peptide hormones. Annu Rev Biochem. 1991;60:229–255. - PubMed
-
- Brenner B.M., Ballermann B.J., Gunning M.E., Zeidel M.L. Diverse biological actions of atrial natriuretic peptide. Physiol Rev. 1990;70:665–699. - PubMed
-
- Inagami T. Atrial natriuretic factor. J Biol Chem. 1989;264:3043–3046. - PubMed
-
- de Bold A.J. Atrial natriuretic factor: a hormone produced by the heart. Science. 1985;230:767–770. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

