Sleep and body temperature in TNFα knockout mice: The effects of sleep deprivation, β3-AR stimulation and exogenous TNFα
- PMID: 31220563
- PMCID: PMC6754767
- DOI: 10.1016/j.bbi.2019.06.022
Sleep and body temperature in TNFα knockout mice: The effects of sleep deprivation, β3-AR stimulation and exogenous TNFα
Abstract
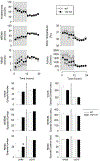
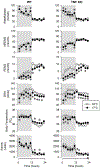
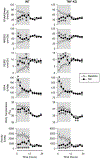
Increased production of pro-inflammatory cytokines is assumed to mediate increased sleep under inflammatory conditions, such as systemic infections or recovery from sleep loss. The role of cytokines in sleep regulation under normal conditions is less clear. In the present study, we investigated the role of endogenous tumor necrosis factor alpha (TNFα) in sleep regulation using TNFα knockout (KO) mice. Under control conditions at thermoneutral ambient temperature, total sleep time did not differ between TNFα KO and wild-type (WT) mice, but TNFα KO mice had increased rapid-eye-movement sleep (REMS), accompanied by decreased motor activity and body temperature. Exposure to 17 °C induced decreases in total sleep time similarly in both genotypes. Sleep deprivation by gentle handling elicited robust rebound increases in non-rapid-eye movement sleep (NREMS), REMS and electroencephalographic (EEG) slow-wave activity (SWA), accompanied by suppressed motor activity and decreased body temperature; there was no significant difference between the responses of WT and KO mice. Systemic injection of the beta3-adrenergic receptor (β3-AR) agonist CL-316,243 induced increases in NREMS and body temperature. The temperature response, but not the sleep effect, was attenuated in the KO animals. Systemic injection of TNFα induced increased NREMS, reduced REMS and biphasic temperature responses in both genotypes. In the KO mice, the NREMS-promoting effects of exogenously administered TNFα was decreased, while REMS suppression was enhanced, and the first, hypothermic, phase of temperature response was attenuated. Overall, TNFα KO mice did not show any deficiency in sleep regulation which suggests that the role of endogenous TNFα in sleep regulation is less pronounced than previously suggested.
Copyright © 2019 Elsevier Inc. All rights reserved.
Figures


References
-
- Arruda AP, Milanski M, Romanatto T, Solon C, Coope A, Alberici LC, Festuccia WT, Hirabara SM, Ropelle E, Curi R, Carvalheira JB, Vercesi AE, Velloso LA, 2010. Hypothalamic actions of tumor necrosis factor αprovide the thermogenic core for the wastage syndrome in cachexia. Endocrinology 151, 683–694. - PubMed
-
- Basheer R, Rainnie DG, Porkka-Heiskanen T, Ramesh V, McCarley RW, 2001. Adenosine, prolonged wakefulness, and A1-activated NF-κB DNA binding in the basal forebrain of the rat. Neuroscience 104, 731–739. - PubMed
-
- Brandt JA, Churchill L, Rehman A, Ellis G, Memet S, Israel A, Krueger JM, 2004. Sleep deprivation increases the activation of nuclear factor kappa B in lateral hypothalamic cells. Brain Res. 1004, 91–97. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

