Misoprostol protects mice against severe Clostridium difficile infection and promotes recovery of the gut microbiota after antibiotic perturbation
- PMID: 31220605
- PMCID: PMC6697607
- DOI: 10.1016/j.anaerobe.2019.06.006
Misoprostol protects mice against severe Clostridium difficile infection and promotes recovery of the gut microbiota after antibiotic perturbation
Abstract
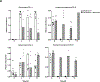
Clostridium difficile infection (CDI) is one of the most common nosocomial infections worldwide and an urgent public health threat. Epidemiological and experimental studies have demonstrated an association between nonsteroidal anti-inflammatory drug (NSAID) exposure and enhanced susceptibility to, and severity of, CDI. NSAIDs target cyclooxygenase enzymes and inhibit the production of prostaglandins (PGs), but the therapeutic potential of exogenous introduction of PGs for the treatment of CDI has not been explored. In this study, we report that treatment with the FDA-approved stable PGE1 analogue, misoprostol, protects mice against C. difficile-associated mortality, intestinal pathology, and CDI-mediated intestinal permeability. Furthermore, we report that the effect of misoprostol on the gastrointestinal tract contributes to increased recovery of the gut microbiota following antibiotic perturbation. Together, these data implicate PGs as an important host-factor associated with recovery to C. difficile-associated disease and demonstrate the potential for misoprostol in the treatment of CDI. Further studies to explore the safety and efficacy of misoprostol treatment of CDI in humans is needed.
Keywords: Clinical pharmacology; Colitis; Microbiome; NSAIDs; Prostaglandins.
Copyright © 2019 Elsevier Ltd. All rights reserved.
Figures
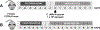
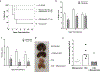
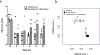

Similar articles
-
Nonsteroidal Anti-inflammatory Drugs Alter the Microbiota and Exacerbate Clostridium difficile Colitis while Dysregulating the Inflammatory Response.mBio. 2019 Jan 8;10(1):e02282-18. doi: 10.1128/mBio.02282-18. mBio. 2019. PMID: 30622186 Free PMC article.
-
Impact of Oral Fidaxomicin Administration on the Intestinal Microbiota and Susceptibility to Clostridium difficile Colonization in Mice.Antimicrob Agents Chemother. 2018 Apr 26;62(5):e02112-17. doi: 10.1128/AAC.02112-17. Print 2018 May. Antimicrob Agents Chemother. 2018. PMID: 29463537 Free PMC article.
-
A Detrimental Role of Immunosuppressive Drug, Dexamethasone, During Clostridium difficile Infection in Association with a Gastrointestinal Microbial Shift.J Microbiol Biotechnol. 2016 Mar;26(3):567-71. doi: 10.4014/jmb.1512.12017. J Microbiol Biotechnol. 2016. PMID: 26809802 Free PMC article.
-
Fecal microbiota transplantation for the treatment of Clostridium difficile infection.J Hosp Med. 2016 Jan;11(1):56-61. doi: 10.1002/jhm.2449. Epub 2015 Sep 7. J Hosp Med. 2016. PMID: 26344412 Free PMC article. Review.
-
Breakthroughs in the treatment and prevention of Clostridium difficile infection.Nat Rev Gastroenterol Hepatol. 2016 Mar;13(3):150-60. doi: 10.1038/nrgastro.2015.220. Epub 2016 Feb 10. Nat Rev Gastroenterol Hepatol. 2016. PMID: 26860266 Review.
Cited by
-
Non-steroidal anti-inflammatory drugs are not associated with increased risk of Clostridioides difficile infection: A propensity-score-matched case-control study.Anaerobe. 2021 Dec;72:102444. doi: 10.1016/j.anaerobe.2021.102444. Epub 2021 Sep 22. Anaerobe. 2021. PMID: 34506930 Free PMC article.
-
New treatment approaches for Clostridioides difficile infections: alternatives to antibiotics and fecal microbiota transplantation.Gut Microbes. 2024 Jan-Dec;16(1):2337312. doi: 10.1080/19490976.2024.2337312. Epub 2024 Apr 9. Gut Microbes. 2024. PMID: 38591915 Free PMC article. Review.
-
Translational Aspects of the Immunology of Clostridioides difficile Infection: Implications for Pediatric Populations.J Pediatric Infect Dis Soc. 2021 Nov 17;10(Supplement_3):S8-S15. doi: 10.1093/jpids/piab089. J Pediatric Infect Dis Soc. 2021. PMID: 34791392 Free PMC article. Review.
-
From Nursery to Nursing Home: Emerging Concepts in Clostridioides difficile Pathogenesis.Infect Immun. 2020 Jun 22;88(7):e00934-19. doi: 10.1128/IAI.00934-19. Print 2020 Jun 22. Infect Immun. 2020. PMID: 32122939 Free PMC article. Review.
-
New Host-Directed Therapeutics for the Treatment of Clostridioides difficile Infection.mBio. 2020 Mar 10;11(2):e00053-20. doi: 10.1128/mBio.00053-20. mBio. 2020. PMID: 32156806 Free PMC article.
References
-
- Kelly CP, LaMont JT Clostridium difficile infection. Annu Rev Med 49 (1998) 375–90. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

