Redox Chemistry in the Genome: Emergence of the [4Fe4S] Cofactor in Repair and Replication
- PMID: 31220976
- PMCID: PMC6590699
- DOI: 10.1146/annurev-biochem-013118-110644
Redox Chemistry in the Genome: Emergence of the [4Fe4S] Cofactor in Repair and Replication
Abstract
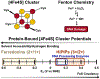
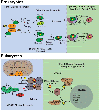
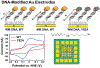
Many DNA-processing enzymes have been shown to contain a [4Fe4S] cluster, a common redox cofactor in biology. Using DNA electrochemistry, we find that binding of the DNA polyanion promotes a negative shift in [4Fe4S] cluster potential, which corresponds thermodynamically to a ∼500-fold increase in DNA-binding affinity for the oxidized [4Fe4S]3+ cluster versus the reduced [4Fe4S]2+ cluster. This redox switch can be activated from a distance using DNA charge transport (DNA CT) chemistry. DNA-processing proteins containing the [4Fe4S] cluster are enumerated, with possible roles for the redox switch highlighted. A model is described where repair proteins may signal one another using DNA-mediated charge transport as a first step in their search for lesions. The redox switch in eukaryotic DNA primases appears to regulate polymerase handoff, and in DNA polymerase δ, the redox switch provides a means to modulate replication in response to oxidative stress. We thus describe redox signaling interactions of DNA-processing [4Fe4S] enzymes, as well as the most interesting potential players to consider in delineating new DNA-mediated redox signaling networks.
Keywords: DNA charge transport; DNA polymerase; DNA primase; base excision repair; iron–sulfur clusters; oxidative stress; redox signaling.
Figures


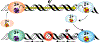




Similar articles
-
The Oxidation State of [4Fe4S] Clusters Modulates the DNA-Binding Affinity of DNA Repair Proteins.J Am Chem Soc. 2017 Sep 13;139(36):12784-12792. doi: 10.1021/jacs.7b07230. Epub 2017 Aug 29. J Am Chem Soc. 2017. PMID: 28817778 Free PMC article.
-
The [4Fe4S] cluster of human DNA primase functions as a redox switch using DNA charge transport.Science. 2017 Feb 24;355(6327):eaag1789. doi: 10.1126/science.aag1789. Science. 2017. PMID: 28232525 Free PMC article.
-
DNA-bound redox activity of DNA repair glycosylases containing [4Fe-4S] clusters.Biochemistry. 2005 Jun 14;44(23):8397-407. doi: 10.1021/bi047494n. Biochemistry. 2005. PMID: 15938629
-
Eukaryotic Base Excision Repair: New Approaches Shine Light on Mechanism.Annu Rev Biochem. 2019 Jun 20;88:137-162. doi: 10.1146/annurev-biochem-013118-111315. Annu Rev Biochem. 2019. PMID: 31220977 Free PMC article. Review.
-
DNA repair glycosylases with a [4Fe-4S] cluster: a redox cofactor for DNA-mediated charge transport?J Inorg Biochem. 2007 Nov;101(11-12):1913-21. doi: 10.1016/j.jinorgbio.2007.05.001. Epub 2007 May 17. J Inorg Biochem. 2007. PMID: 17599416 Free PMC article. Review.
Cited by
-
The Influence of 2'-Deoxyguanosine Lesions on the Electronic Properties of OXOG:::C Base Pairs in Ds-DNA: A Comparative Analysis of Theoretical Studies.Molecules. 2024 Aug 8;29(16):3756. doi: 10.3390/molecules29163756. Molecules. 2024. PMID: 39202837 Free PMC article. Review.
-
Outlining the Complex Pathway of Mammalian Fe-S Cluster Biogenesis.Trends Biochem Sci. 2020 May;45(5):411-426. doi: 10.1016/j.tibs.2020.02.001. Epub 2020 Mar 6. Trends Biochem Sci. 2020. PMID: 32311335 Free PMC article. Review.
-
Helicases FANCJ, RTEL1 and BLM Act on Guanine Quadruplex DNA in Vivo.Genes (Basel). 2019 Oct 31;10(11):870. doi: 10.3390/genes10110870. Genes (Basel). 2019. PMID: 31683575 Free PMC article. Review.
-
Iron-sulfur protein odyssey: exploring their cluster functional versatility and challenging identification.Metallomics. 2024 May 2;16(5):mfae025. doi: 10.1093/mtomcs/mfae025. Metallomics. 2024. PMID: 38744662 Free PMC article. Review.
-
A Highly Conserved Iron-Sulfur Cluster Assembly Machinery between Humans and Amoeba Dictyostelium discoideum: The Characterization of Frataxin.Int J Mol Sci. 2020 Sep 17;21(18):6821. doi: 10.3390/ijms21186821. Int J Mol Sci. 2020. PMID: 32957566 Free PMC article.
References
-
- Beinert H, Holm RH, Munck E. 1997. Iron-sulfur clusters: Nature’s modular, multipurpose structures. Science 277:653–59 - PubMed
-
- Rees DC, Howard JB. 2003. The interface between the biological and inorganic worlds: Iron-sulfur metalloclusters. Science 300:929–31 - PubMed
-
- Meyer J 2008. Iron-sulfur protein folds, iron-sulfur chemistry, and evolution. J. Biol. Inorg. Chem 13:157–70 - PubMed
-
- Dey A, Jenney FE, Adams MWW, Babini E, Takahashi Y, et al. 2007. Solvent tuning of electrochemical potentials in the active sites of HiPIP versus ferredoxin. Science 318:1464–68 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

