Eukaryotic Base Excision Repair: New Approaches Shine Light on Mechanism
- PMID: 31220977
- PMCID: PMC8956022
- DOI: 10.1146/annurev-biochem-013118-111315
Eukaryotic Base Excision Repair: New Approaches Shine Light on Mechanism
Abstract
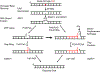
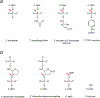
Genomic DNA is susceptible to endogenous and environmental stresses that modify DNA structure and its coding potential. Correspondingly, cells have evolved intricate DNA repair systems to deter changes to their genetic material. Base excision DNA repair involves a number of enzymes and protein cofactors that hasten repair of damaged DNA bases. Recent advances have identified macromolecular complexes that assemble at the DNA lesion and mediate repair. The repair of base lesions generally requires five enzymatic activities: glycosylase, endonuclease, lyase, polymerase, and ligase. The protein cofactors and mechanisms for coordinating the sequential enzymatic steps of repair are being revealed through a range of experimental approaches. We discuss the enzymes and protein cofactors involved in eukaryotic base excision repair, emphasizing the challenge of integrating findings from multiple methodologies. The results provide an opportunity to assimilate biochemical findings with cell-based assays to uncover new insights into this deceptively complex repair pathway.
Keywords: DNA polymerase; genome stability; mechanism; mutagenesis; repair; structure.
Figures
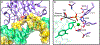
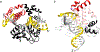

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

