Performance and impact of GeneXpert MTB/RIF® and Loopamp MTBC Detection Kit® assays on tuberculosis case detection in Madagascar
- PMID: 31221109
- PMCID: PMC6585144
- DOI: 10.1186/s12879-019-4198-6
Performance and impact of GeneXpert MTB/RIF® and Loopamp MTBC Detection Kit® assays on tuberculosis case detection in Madagascar
Abstract
Background: Tuberculosis rapid molecular assays, including GeneXpert MTB/RIF® and Loopamp MTBC Detection Kit®, are highly sensitive and specific. Such performance does not automatically translate in improved disease control and highly depends on their use, local epidemiology and the diagnostic algorithms they're implemented within. We evaluate the performance of both assays and assess their impact on additional cases notification when implemented within WHO recommended tuberculosis diagnostic algorithms in Madagascar.
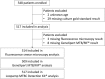
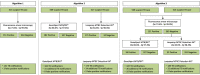
Methods: Five hundred forty eight presumptive pulmonary tuberculosis patients were prospectively recruited between November 2013 and December 2014 in Antananarivo, Madagascar, a high TB incidence sub-Saharan African urban setting. Both molecular assays were evaluated as first line or add-on testing following negative smear microscopy. Based on locally defined assay performance characteristics we measure the impact of both assays and WHO-recommended diagnostic algorithms on additional tuberculosis case notifications.
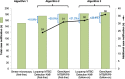
Results: High sensitivity and specificity was confirmed for both GeneXpert MTB/RIF® (86.6% (95% CI 81.1-90.7%) and 97.4% (95% CI 94.9-98.8%)) and Loopamp MTBC Detection Kit® (84.6% (95% CI 78.9-89.0%) and 98.4% (95% CI 96.2-99.4%)). Implementation of GeneXpert MTB/RIF® and Loopamp MTBC Detection Kit® increased tuberculosis diagnostic algorithms sensitivity from 73.6% (95% CI 67.1-79.3%) up to 88.1% (95% CI 82.8-91.9%). This increase was highest when molecular assays were used as add-on testing following negative smear microscopy. As add-on testing, GeneXpert MTB/RIF® and Loopamp MTBC Detection Kit® respectively improved case detection by 23.8 and 21.2% (p < 0.05).
Conclusion: Including GeneXpert MTB/RIF® or Loopamp MTBC Detection Kit® molecular assays for TB detection on sputum samples from presumptive TB cases can significantly increase case notification in TB diagnostic centers. The TB case detection rate is further increased when those tests are use as second-line follow-on testing following negative smear microscopy results. A country wide scale-up and digital integration of molecular-based TB diagnosis assays shows promises for TB control in Madagascar.
Keywords: Case detection; GeneXpert; Loop-mediated isothermal amplification; Molecular diagnostics; Tuberculosis.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
-
- WHO: Global TB Report. In.; 2018.
-
- Nagai K, Horita N, Yamamoto M, Tsukahara T, Nagakura H, Tashiro K, Shibata Y, Watanabe H, Nakashima K, Ushio R, et al. Diagnostic test accuracy of loop-mediated isothermal amplification assay for mycobacterium tuberculosis: systematic review and meta-analysis. Sci Rep. 2016;6:39090. doi: 10.1038/srep39090. - DOI - PMC - PubMed
-
- WHO: The use of loop-mediated isothermal amplification (TB-LAMP) for the diagnosis of pulmonary tuberculosis: policy guidance. World Health Organization 2016. - PubMed
-
- Initiative GL: GLI model TB diagnostic algorithms. In.; 2017.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

