Metabolic consequences of cystinuria
- PMID: 31221135
- PMCID: PMC6585015
- DOI: 10.1186/s12882-019-1417-8
Metabolic consequences of cystinuria
Abstract
Background: Cystinuria is an inherited disorder of renal amino acid transport that causes recurrent nephrolithiasis and significant morbidity in humans. It has an incidence of 1 in 7000 worldwide making it one of the most common genetic disorders in man. We phenotypically characterized a mouse model of cystinuria type A resultant from knockout of Slc3a1.
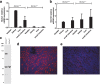
Methods: Knockout of Slc3a1 at RNA and protein levels was evaluated using real-time quantitative PCR and immunofluorescence. Slc3a1 knockout mice were placed on normal or breeder chow diets and evaluated for cystine stone formation over time suing x-ray analysis, and the development of kidney injury by measuring injury biomarkers. Kidney injury was also evaluated via histologic analysis. Amino acid levels were measured in the blood of mice using high performance liquid chromatography. Liver glutathione levels were measured using a luminescent-based assay.
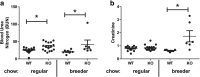
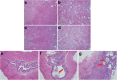
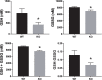
Results: We confirmed knockout of Slc3a1 at the RNA level, while Slc7a9 RNA representing the co-transporter was preserved. As expected, we observed bladder stone formation in Slc3a1-/- mice. Male Slc3a1-/- mice exhibited lower weights compared to Slc3a1+/+. Slc3a1-/- mice on a regular diet demonstrated elevated blood urea nitrogen (BUN) without elevation of serum creatinine. However, placing the knockout animals on a breeder chow diet, containing a higher cystine concentration, resulted in the development of elevation of both BUN and creatinine indicative of more severe chronic kidney disease. Histological examination revealed that these dietary effects resulted in worsened kidney tubular obstruction and interstitial inflammation as well as worsened bladder inflammation. Cystine is a precursor for the antioxidant molecule glutathione, so we evaluated glutathione levels in the livers of Slc3a1-/- mice. We found significantly lowered levels of both reduced and total glutathione in the knockout animals.
Conclusions: Our results suggest that that diet can affect the development and progression of chronic kidney disease in an animal model of cystinuria, which may have important implications for patients with this disease. Additionally, reduced glutathione may predispose those with cystinuria to injury caused by oxidative stress. Word count: 327.
Keywords: Chronic kidney disease; Cystine; Cystinuria; Kidney stones; Nephrolithiasis.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


Comment in
-
Re: Metabolic Consequences of Cystinuria.J Urol. 2019 Dec;202(6):1091-1093. doi: 10.1097/JU.0000000000000557. Epub 2019 Sep 18. J Urol. 2019. PMID: 31532293 No abstract available.
References
-
- Chillaron J, Font-Llitjos M, Fort J, Zorzano A, Goldfarb DS, Nunes V, et al. Pathophysiology and treatment of cystinuria. Nat Rev Nephrol. 2010;6(7):424–434. - PubMed
-
- Sakhaee K. Pathogenesis and medical management of cystinuria. SeminNephrol. 1996;16(5):435–447. - PubMed
-
- Calonge MJ, Gasparini P, Chillaron J, Chillon M, Gallucci M, Rousaud F, et al. Cystinuria caused by mutations in rBAT, a gene involved in the transport of cystine. NatGenet. 1994;6(4):420–425. - PubMed
-
- Feliubadalo L, Font M, Purroy J, Rousaud F, Estivill X, Nunes V, et al. Non-type I cystinuria caused by mutations in SLC7A9, encoding a subunit (b(o,+)AT) of rBAT. Nat Genet. 1999;23(1):52–57. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

