Disposition and effect of intra-articularly administered dexamethasone on lipopolysaccharide induced equine synovitis
- PMID: 31221173
- PMCID: PMC6585085
- DOI: 10.1186/s13028-019-0464-2
Disposition and effect of intra-articularly administered dexamethasone on lipopolysaccharide induced equine synovitis
Abstract
Background: Dexamethasone is used for the intra-articular route of administration in management of aseptic arthritis in horses. Despite its widespread use there is very little quantitative data of the disposition and response to dexamethasone. The aim of this study was to investigate and describe the synovial fluid and plasma dexamethasone concentration over time and to explore the relation between synovial fluid concentration and response using clinical endpoints as response biomarkers after IA injection of dexamethasone disodium salt solution in an equine model of synovitis.
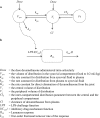
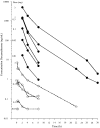
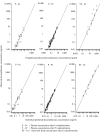
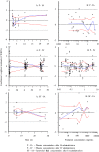
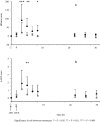
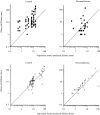
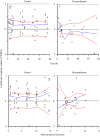
Results: Inflammation was induced in the radiocarpal joint of six horses by injection of 2 ng lipopolysaccharide (LPS). Two hours later either saline or dexamethasone was injected in the same joint in a two treatment cross over design. Each horse was treated once with one of the six doses dexamethasone used (0.01, 0.03, 0.1, 0.3, 1 or 3 mg) and once with saline. Dexamethasone was quantified by means of UHPLC-MS/MS. Dexamethasone disposition was characterised by means of a non-linear mixed effects model. Lameness was evaluated both objectively with an inertial sensor based system and subjectively scored using a numerical scale (0-5). Joint circumference, skin temperature over the joint and rectal temperature were also recorded. The LPS-challenge induced lameness in all horses with high inter-individual variability. Dexamethasone significantly decreased lameness compared with saline. Other variables were not statistically significant different between treatments. Objective lameness scoring was the most sensitive method used in this study to evaluate the lameness response. A pharmacokinetic/pharmacodynamic model was successfully fitted to experimental dexamethasone and lameness data. The model allowed characterization of the dexamethasone synovial fluid concentration-time course, the systemic exposure to dexamethasone after intra-articular administration and the concentration-response relation in an experimental model of synovitis.
Conclusions: The quantitative data improve the understanding of the pharmacology of dexamethasone and might serve as input for future experiments and possibly contribute to maintain integrity of equine sports.
Keywords: Corticosteroids; Pharmacodynamics; Pharmacokinetics; Quantitative pharmacology.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
-
- Neundorf RH, Lowerison MB, Cruz AM, Thomason JJ, McEwen BJ, Hurtig MB. Determination of the prevalence and severity of metacarpophalangeal joint osteoarthritis in Thoroughbred racehorses via quantitative macroscopic evaluation. Am J Vet Res. 2010;71:1284–1293. doi: 10.2460/ajvr.71.11.1284. - DOI - PubMed
-
- Kay AT, Bolt DM, Ishihara A, Rajala-Schultz PJ, Bertone AL. Anti-inflammatory and analgesic effects of intra-articular injection of triamcinolone acetonide, mepivacaine hydrochloride, or both on lipopolysaccharide-induced lameness in horses. Am J Vet Res. 2008;69:1646–1654. doi: 10.2460/ajvr.69.12.1646. - DOI - PubMed
-
- Salles-Gomes TL, Canola JC, Almeida PE, Canola PA, Souza AH. The effects of intra-articular and intravenous dexamethasone tereoxiacetate administration in horses with intercarpal arthritis induced by injection of Escherichia coli lipopolysaccharide. Ars Vet. 2003;19:241–245.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

