Discovery of an intrasubunit nicotinic acetylcholine receptor-binding site for the positive allosteric modulator Br-PBTC
- PMID: 31221718
- PMCID: PMC6690704
- DOI: 10.1074/jbc.RA118.006253
Discovery of an intrasubunit nicotinic acetylcholine receptor-binding site for the positive allosteric modulator Br-PBTC
Abstract
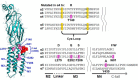
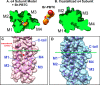
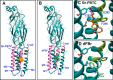
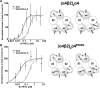
Nicotinic acetylcholine receptor (nAChR) ligands that lack agonist activity but enhance activation in the presence of an agonist are called positive allosteric modulators (PAMs). nAChR PAMs have therapeutic potential for the treatment of nicotine addiction and several neuropsychiatric disorders. PAMs need to be selectively targeted toward certain nAChR subtypes to tap this potential. We previously discovered a novel PAM, (R)-7-bromo-N-(piperidin-3-yl)benzo[b]thiophene-2-carboxamide (Br-PBTC), which selectively potentiates the opening of α4β2*, α2β2*, α2β4*, and (α4β4)2α4 nAChRs and reactivates some of these subtypes when desensitized (* indicates the presence of other subunits). We located the Br-PBTC-binding site through mutagenesis and docking in α4. The amino acids Glu-282 and Phe-286 near the extracellular domain on the third transmembrane helix were found to be crucial for Br-PBTC's PAM effect. E282Q abolishes Br-PBTC potentiation. Using (α4E282Qβ2)2α5 nAChRs, we discovered that the trifluoromethylated derivatives of Br-PBTC can potentiate channel opening of α5-containing nAChRs. Mutating Tyr-430 in the α5 M4 domain changed α5-selectivity among Br-PBTC derivatives. There are two kinds of α4 subunits in α4β2 nAChRs. Primary α4 forms an agonist-binding site with another β2 subunit. Accessory α4 forms an agonist-binding site with another α4 subunit. The pharmacological effect of Br-PBTC depends both on its own and agonists' occupancy of primary and accessory α4 subunits. Br-PBTC reactivates desensitized (α4β2)2α4 nAChRs. Its full efficacy requires intact Br-PBTC sites in at least one accessory and one primary α4 subunit. PAM potency increases with higher occupancy of the agonist sites. Br-PBTC and its derivatives should prove useful as α subunit-selective nAChR PAMs.
Keywords: allosteric regulation; drug design; molecular docking; nicotinic acetylcholine receptors (nAChR); positive allosteric modulator; receptor desensitization.
© 2019 Norleans et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures





References
-
- Williams D. K., Wang J., and Papke R. L. (2011) Investigation of the molecular mechanism of the α7 nicotinic acetylcholine receptor positive allosteric modulator PNU-120596 provides evidence for two distinct desensitized states. Mol. Pharmacol. 80, 1013–1032 10.1124/mol.111.074302 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

