Soybean (Glycine max L.) triacylglycerol lipase GmSDP1 regulates the quality and quantity of seed oil
- PMID: 31222045
- PMCID: PMC6586785
- DOI: 10.1038/s41598-019-45331-8
Soybean (Glycine max L.) triacylglycerol lipase GmSDP1 regulates the quality and quantity of seed oil
Abstract
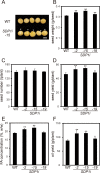
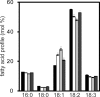
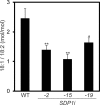
Seeds of soybean (Glycine max L.) are a major source of plant-derived oils. In the past, improvements have been made in the quantity and quality of seed oil. Triacylglycerols (TAGs) are the principal components of soybean seed oil, and understanding the metabolic regulation of TAGs in soybean seeds is essential. Here, we identified four soybean genes encoding TAG lipases, designated as SUGAR DEPENDENT1-1 (GmSDP1-1), GmSDP1-2, GmSDP1-3 and GmSDP1-4; these are homologous to Arabidopsis thaliana SDP1 (AtSDP1). To characterize the function of these genes during grain filling, transgenic lines of soybean were generated via RNA interference to knockdown the expression of all four GmSDP1 genes. The seed oil content of the transgenic soybean lines was significantly increased compared with the wild type (WT). Additionally, fatty acid profiles of the WT and transgenic soybean lines were altered; the content of linoleic acid, a major fatty acid in soybean seeds, was significantly reduced, whereas that of oleic acid was increased in transgenic soybean seeds compared with the WT. Substrate specificity experiments showed that TAG lipase preferentially cleaved oleic acid than linoleic acid in the oil body membrane in WT soybean. This study demonstrates that the GmSDP1 proteins regulate both the TAG content and fatty acid composition of soybean seeds during grain filling. These results provide a novel strategy for improving both the quantity and quality of soybean seed oil.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

