Brain leptin reduces liver lipids by increasing hepatic triglyceride secretion and lowering lipogenesis
- PMID: 31222048
- PMCID: PMC6586634
- DOI: 10.1038/s41467-019-10684-1
Brain leptin reduces liver lipids by increasing hepatic triglyceride secretion and lowering lipogenesis
Abstract
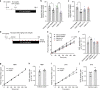
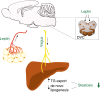
Hepatic steatosis develops when lipid influx and production exceed the liver's ability to utilize/export triglycerides. Obesity promotes steatosis and is characterized by leptin resistance. A role of leptin in hepatic lipid handling is highlighted by the observation that recombinant leptin reverses steatosis of hypoleptinemic patients with lipodystrophy by an unknown mechanism. Since leptin mainly functions via CNS signaling, we here examine in rats whether leptin regulates hepatic lipid flux via the brain in a series of stereotaxic infusion experiments. We demonstrate that brain leptin protects from steatosis by promoting hepatic triglyceride export and decreasing de novo lipogenesis independently of caloric intake. Leptin's anti-steatotic effects are generated in the dorsal vagal complex, require hepatic vagal innervation, and are preserved in high-fat-diet-fed rats when the blood brain barrier is bypassed. Thus, CNS leptin protects from ectopic lipid accumulation via a brain-vagus-liver axis and may be a therapeutic strategy to ameliorate obesity-related steatosis.
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
Leptin increases hepatic triglyceride export via a vagal mechanism in humans.Cell Metab. 2022 Nov 1;34(11):1719-1731.e5. doi: 10.1016/j.cmet.2022.09.020. Epub 2022 Oct 10. Cell Metab. 2022. PMID: 36220067 Clinical Trial.
-
Liver sympathetic denervation reverses obesity-induced hepatic steatosis.J Physiol. 2019 Sep;597(17):4565-4580. doi: 10.1113/JP277994. Epub 2019 Jul 26. J Physiol. 2019. PMID: 31278754 Free PMC article.
-
Leptin limits hepatic lipid accumulation and inflammation via vagal activation of the JAK2-STAT3/AMPK pathway.J Nutr Biochem. 2024 Dec;134:109748. doi: 10.1016/j.jnutbio.2024.109748. Epub 2024 Aug 24. J Nutr Biochem. 2024. PMID: 39186956
-
Metabolic-associated fatty liver disease and lipoprotein metabolism.Mol Metab. 2021 Aug;50:101238. doi: 10.1016/j.molmet.2021.101238. Epub 2021 Apr 20. Mol Metab. 2021. PMID: 33892169 Free PMC article. Review.
-
Mechanisms of hepatic triglyceride accumulation in non-alcoholic fatty liver disease.J Gastroenterol. 2013 Apr;48(4):434-41. doi: 10.1007/s00535-013-0758-5. Epub 2013 Feb 9. J Gastroenterol. 2013. PMID: 23397118 Free PMC article. Review.
Cited by
-
Role of Leptin in the Digestive System.Front Pharmacol. 2021 Apr 12;12:660040. doi: 10.3389/fphar.2021.660040. eCollection 2021. Front Pharmacol. 2021. PMID: 33935782 Free PMC article. Review.
-
Neuronal control of peripheral nutrient partitioning.Diabetologia. 2020 Apr;63(4):673-682. doi: 10.1007/s00125-020-05104-9. Epub 2020 Feb 7. Diabetologia. 2020. PMID: 32030470 Review.
-
Evaluation of Hepatic Glucose and Palmitic Acid Metabolism in Rodents on High-Fat Diet Using Deuterium Metabolic Imaging.J Magn Reson Imaging. 2025 Feb;61(2):958-967. doi: 10.1002/jmri.29437. Epub 2024 May 9. J Magn Reson Imaging. 2025. PMID: 38721871 Free PMC article.
-
Liver diseases: epidemiology, causes, trends and predictions.Signal Transduct Target Ther. 2025 Feb 5;10(1):33. doi: 10.1038/s41392-024-02072-z. Signal Transduct Target Ther. 2025. PMID: 39904973 Free PMC article. Review.
-
Nutrient infusion in the dorsal vagal complex controls hepatic lipid and glucose metabolism in rats.iScience. 2021 Mar 26;24(4):102366. doi: 10.1016/j.isci.2021.102366. eCollection 2021 Apr 23. iScience. 2021. PMID: 33870148 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
- 634413/EC | Horizon 2020 Framework Programme (EU Framework Programme for Research and Innovation H2020)/International
- P30 DK020541/DK/NIDDK NIH HHS/United States
- P30 DK026687/DK/NIDDK NIH HHS/United States
- P26766/Austrian Science Fund (Fonds zur Förderung der Wissenschaftlichen Forschung)/International
- P 30830/FWF_/Austrian Science Fund FWF/Austria
LinkOut - more resources
Full Text Sources
Medical

