Innovative mouse model mimicking human-like features of spinal cord injury: efficacy of Docosahexaenoic acid on acute and chronic phases
- PMID: 31222077
- PMCID: PMC6586623
- DOI: 10.1038/s41598-019-45037-x
Innovative mouse model mimicking human-like features of spinal cord injury: efficacy of Docosahexaenoic acid on acute and chronic phases
Erratum in
-
Publisher Correction: Innovative mouse model mimicking human-like features of spinal cord injury: efficacy of Docosahexaenoic acid on acute and chronic phases.Sci Rep. 2019 Nov 14;9(1):17043. doi: 10.1038/s41598-019-53787-x. Sci Rep. 2019. PMID: 31728071 Free PMC article.
Abstract
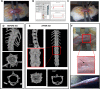
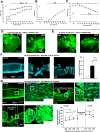
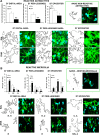
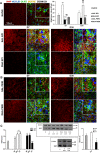
Traumatic spinal cord injury has dramatic consequences and a huge social impact. We propose a new mouse model of spinal trauma that induces a complete paralysis of hindlimbs, still observable 30 days after injury. The contusion, performed without laminectomy and deriving from the pressure exerted directly on the bone, mimics more closely many features of spinal injury in humans. Spinal cord was injured at thoracic level 10 (T10) in adult anesthetized female CD1 mice, mounted on stereotaxic apparatus and connected to a precision impactor device. Following severe injury, we evaluated motor and sensory functions, and histological/morphological features of spinal tissue at different time points. Moreover, we studied the effects of early and subchronic administration of Docosahexaenoic acid, investigating functional responses, structural changes proximal and distal to the lesion in primary and secondary injury phases, proteome modulation in injured spinal cord. Docosahexaenoic acid was able i) to restore behavioural responses and ii) to induce pro-regenerative effects and neuroprotective action against demyelination, apoptosis and neuroinflammation. Considering the urgent health challenge represented by spinal injury, this new and reliable mouse model together with the positive effects of docosahexaenoic acid provide important translational implications for promising therapeutic approaches for spinal cord injuries.
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Blockade of IL-6 signaling by MR16-1 inhibits reduction of docosahexaenoic acid-containing phosphatidylcholine levels in a mouse model of spinal cord injury.Neuroscience. 2014 Jun 6;269:1-10. doi: 10.1016/j.neuroscience.2014.03.012. Epub 2014 Mar 20. Neuroscience. 2014. PMID: 24657456
-
In vivo PET imaging of the neuroinflammatory response in rat spinal cord injury using the TSPO tracer [(18)F]GE-180 and effect of docosahexaenoic acid.Eur J Nucl Med Mol Imaging. 2016 Aug;43(9):1710-22. doi: 10.1007/s00259-016-3391-8. Epub 2016 May 7. Eur J Nucl Med Mol Imaging. 2016. PMID: 27154521 Free PMC article.
-
Effects of early surgical decompression on functional and histological outcomes after severe experimental thoracic spinal cord injury.J Neurosurg Spine. 2017 Jan;26(1):62-75. doi: 10.3171/2016.6.SPINE16343. Epub 2016 Sep 16. J Neurosurg Spine. 2017. PMID: 27636866
-
Omega-3 fatty acids and neurological injury.Prostaglandins Leukot Essent Fatty Acids. 2007 Nov-Dec;77(5-6):295-300. doi: 10.1016/j.plefa.2007.10.021. Epub 2007 Nov 26. Prostaglandins Leukot Essent Fatty Acids. 2007. PMID: 18036801 Review.
-
Degenerative and spontaneous regenerative processes after spinal cord injury.J Neurotrauma. 2006 Mar-Apr;23(3-4):264-80. doi: 10.1089/neu.2006.23.263. J Neurotrauma. 2006. PMID: 16629615 Review.
Cited by
-
Revealing the Therapeutic Potential of Botulinum Neurotoxin Type A in Counteracting Paralysis and Neuropathic Pain in Spinally Injured Mice.Toxins (Basel). 2020 Jul 31;12(8):491. doi: 10.3390/toxins12080491. Toxins (Basel). 2020. PMID: 32751937 Free PMC article.
-
Integrated proteomic and metabolomic profiling of lymph after trauma-induced hypercoagulopathy and antithrombotic therapy.Thromb J. 2024 Jul 10;22(1):59. doi: 10.1186/s12959-024-00634-3. Thromb J. 2024. PMID: 38987792 Free PMC article.
-
Xeomin®, a Commercial Formulation of Botulinum Neurotoxin Type A, Promotes Regeneration in a Preclinical Model of Spinal Cord Injury.Toxins (Basel). 2023 Mar 28;15(4):248. doi: 10.3390/toxins15040248. Toxins (Basel). 2023. PMID: 37104185 Free PMC article.
-
Positive Effects of (+)-Epicatechin on Traumatic Spinal Cord Injury Recovery.Biomolecules. 2025 Jun 14;15(6):869. doi: 10.3390/biom15060869. Biomolecules. 2025. PMID: 40563509 Free PMC article.
-
Botulinum Toxin and Neuronal Regeneration after Traumatic Injury of Central and Peripheral Nervous System.Toxins (Basel). 2020 Jul 2;12(7):434. doi: 10.3390/toxins12070434. Toxins (Basel). 2020. PMID: 32630737 Free PMC article. Review.
References
-
- Stokes BT, Jakeman LB. Experimental modelling of human spinal cord injury: a model that crosses the species barrier and mimics the spectrum of human cytopathology. Spinal Cord. 2002;40:101–9. - PubMed
-
- Talac R, et al. Animal models of spinal cord injury for evaluation of tissue engineering treatment strategies. Biomaterials. 2004;25:1505–10. - PubMed
-
- Kundi S, Bicknell R, Ahmed Z. Spinal Cord Injury: Current mammalian models. American Journal of Neuroscience. 2013;4(1):1–12.
-
- Kundi S, Bicknell R, Ahmed Z. The role of angiogenic and wound-healing factors after spinal cord injury in mammals. Neurosci Res. 2013;76:1–9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

