Structural Design and Data Requirements for Simulation Modelling in HIV/AIDS: A Narrative Review
- PMID: 31222521
- PMCID: PMC6711792
- DOI: 10.1007/s40273-019-00817-1
Structural Design and Data Requirements for Simulation Modelling in HIV/AIDS: A Narrative Review
Abstract
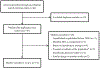
Born out of a necessity for fiscal sustainability, simulation modeling is playing an increasingly prominent role in setting priorities for combination implementation strategies for HIV treatment and prevention globally. The design of a model and the data inputted into it are central factors in ensuring credible inferences. We executed a narrative review of a set of dynamic HIV transmission models to comprehensively synthesize and compare the structural design and the quality of evidence used to support each model. We included 19 models representing both generalized and concentrated epidemics, classified as compartmental, agent-based, individual-based microsimulation or hybrid in our review. We focused on four structural components (population construction; model entry, exit and HIV care engagement; HIV disease progression; and the force of HIV infection), and two analytical components (model calibration/validation; and health economic evaluation, including uncertainty analysis). While the models we reviewed focused on a variety of individual interventions and their combinations, their structural designs were relatively homogenous across three of the four focal components, with key structural elements influenced by model type and epidemiological context. In contrast, model entry, exit and HIV care engagement tended to differ most across models, with some health system interactions-particularly HIV testing-not modeled explicitly in many contexts. The quality of data used in the models and the transparency with which the data was presented differed substantially across model components. Representative and high-quality data on health service delivery were most commonly not accessed or were unavailable. The structure of an HIV model should ideally fit its epidemiological context and be able to capture all efficacious treatment and prevention services relevant to a robust combination implementation strategy. Developing standardized guidelines on evidence syntheses for health economic evaluation would improve transparency and help prioritize data collection to reduce decision uncertainty.
Conflict of interest statement
Conflicts of interest
Xiao Zang, Emanuel Krebs, Linwei Wang, Brandon DL Marshall, Reuben Granich, Bruce R Schackman, Julio SG Montaner, and Bohdan Nosyk have no conflicts of interest to report
Figures






References
-
- Kates J, Wexler A, Lief E, UNAIDS. Donor Government Funding for HIV in Low- and Middle-Income Countries in 2016. 2017. [cited 2017 Aug 20]; Available from: http://www.unaids.org/sites/default/files/media_asset/20170721_Kaiser_Do...
-
- UNAIDS. HIV investments. 2016. [cited 2017 July 24]; Available from: http://www.unaids.org/sites/default/files/media_asset/HIV_investments_Sn...
-
- Jones A, Cremin I, Abdullah F, Idoko J, Cherutich P, Kilonzo N, et al. Transformation of HIV from pandemic to low-endemic levels: a public health approach to combination prevention. Lancet. 2014. July 19;384(9939):272–9. - PubMed
-
- Garnett GP, Cousens S, Hallett TB, Steketee R, Walker N. Mathematical models in the evaluation of health programmes. Lancet. 2011. August 06;378(9790):515–25. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

