Bcl-2/Bcl-xL inhibition predominantly synergistically enhances the anti-neoplastic activity of a low-dose CUSP9 repurposed drug regime against glioblastoma
- PMID: 31222722
- PMCID: PMC6715605
- DOI: 10.1111/bph.14773
Bcl-2/Bcl-xL inhibition predominantly synergistically enhances the anti-neoplastic activity of a low-dose CUSP9 repurposed drug regime against glioblastoma
Abstract
Background and purpose: Drug repurposing represents a promising approach to safely accelerate the clinical application of therapeutics with anti-cancer activity. In this study, we examined whether inhibition of the anti-apoptotic Bcl-2 family proteins Bcl-2 and Bcl-xL enhances the biological effects of the repurposed CUSP9 regimen in an in vitro setting of glioblastoma.
Experimental approach: We applied 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide assays to assess cellular proliferation. Annexin V/propidium iodide and tetramethylrhodamine, ethyl ester staining were used to examine apoptosis. Western blotting, RT-PCR, and specific knockdown experiments using siRNA were employed to examine molecular mechanisms of action.
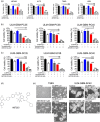
Key results: Bcl-2/Bcl-xL inhibition exerted synergistic anti-proliferative effects across established, primary cultured, and stem-like glioblastoma cells when combined with CUSP9 which had been reduced to only one tenth of its proposed original concentration (CUSP9-LD). The combination treatment also led to enhanced apoptosis with loss of mitochondrial membrane potential and activation of caspases. On the molecular level, CUSP9-LD counteracted ABT263-mediated up-regulation of Mcl-1. Silencing of Mcl-1 enhanced ABT263-mediated apoptosis which indicates that down-regulation of Mcl-1 is crucial for the induction of cell death by the combination treatment.
Conclusion and implications: These data suggest that Bcl-2/Bcl-xL inhibition enhances the susceptibility of glioblastoma cells towards CUSP9, allowing dramatic dose reduction and potentially decreased toxicity when applied clinically. A clinical trial involving the original CUSP doses (CUSP9v3) is currently ongoing in our institution (NCT02770378). The Bcl-2/Bcl-xL inhibitor ABT263 is in clinical trials and might represent a valuable adjunct to the original CUSP.
© 2019 The British Pharmacological Society.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

